Question: Imagine two solutions with the same concentration and the same boiling point, but one has benzene as the solvent and the other has carbon
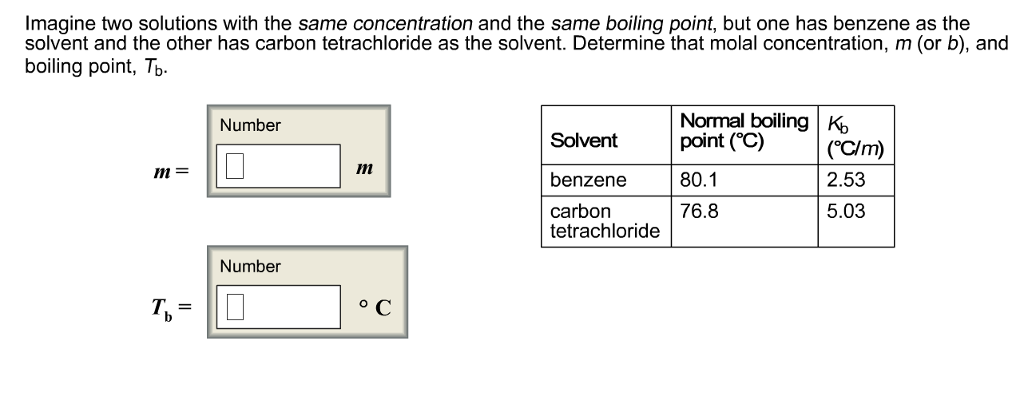
Imagine two solutions with the same concentration and the same boiling point, but one has benzene as the solvent and the other has carbon tetrachloride as the solvent. Determine that molal concentration, m (or b), and boiling point, Tb. m= T= Number Number m C Solvent benzene carbon tetrachloride Normal boiling K point (C) 80.1 76.8 (C/m) 2.53 5.03
Step by Step Solution
3.38 Rating (157 Votes )
There are 3 Steps involved in it
Answer Tb mkb ATb Change in boiling point Boiling point after addition of soluteTp Normal bo... View full answer

Get step-by-step solutions from verified subject matter experts


