Question: In 1765, Benjamin Franklin described experiments that he had done on Clapham Pond near London. He observed that a teaspoon of oil (2.5 cm 3
In 1765, Benjamin Franklin described experiments that he had done on Clapham Pond near London. He observed that a teaspoon of oil (2.5 cm3) placed on the water surface would calm the surface up to 2000 m2. Assuming the oil was oleic acid (below, left image) with a molar mass of 282 g/mol and a density of 0.895 g/cm3, calculate the thickness of the monolayer (nm) and the area per molecule (ao, nm2/molecule). Based on ao and the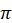
A isotherm for oleic acid (below, right image), what phase is the oleic acid monolayer at pond's air-water interface?
I
Step by Step Solution
There are 3 Steps involved in it
Part a Volume of oleic acid V 45 cm3 x 106 m3cm3 V 45 x 106 m3 Area of surface A 2 ... View full answer

Get step-by-step solutions from verified subject matter experts


