Question: In a fixed - volume batch reactor, reactant A decomposes according to the following homogeneous reaction equation: A - > P The concentration of A
In a fixedvolume batch reactor, reactant A decomposes according to the following homogeneous reaction equation: A P
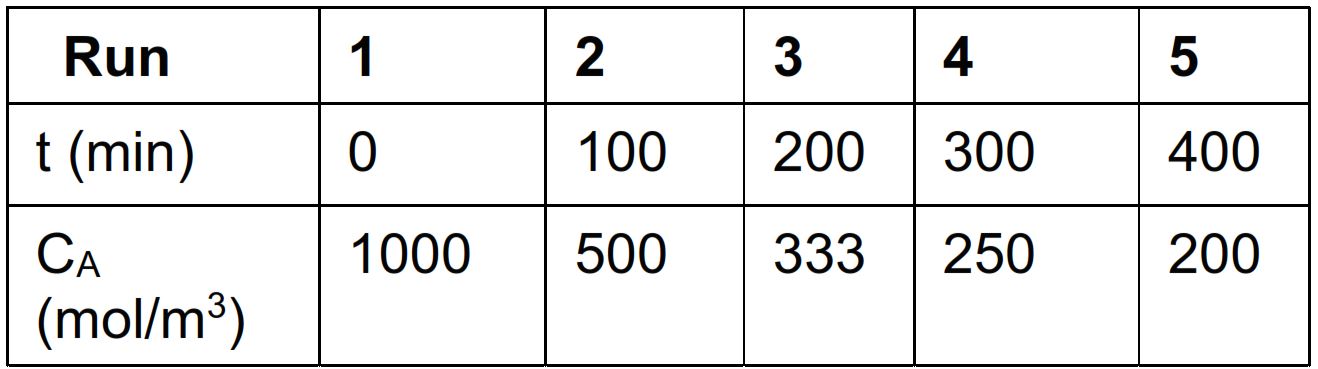
The concentration of A in the reactor CA measured at various times t is presented as follows:
tableRuntmintableCAmolm
Determine the reaction order and the value of the reaction rate constant kA using the method of
a Integral
b PartTime
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


