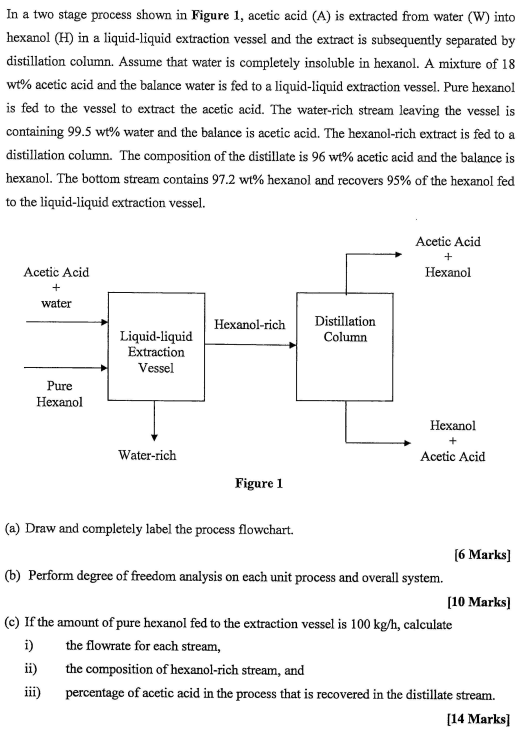
Question: In a two stage process shown in Figure 1 , acetic acid ( A ) is extracted from water ( W ) into hexanol (
In a two stage process shown in Figure acetic acid A is extracted from water W into hexanol in a liquidliquid extraction vessel and the extract is subsequently separated by distillation column. Assume that water is completely insoluble in hexanol. A mixture of acetic acid and the balance water is fed to a liquidliquid extraction vessel. Pure hexanol is fed to the vessel to extract the acetic acid. The waterrich stream leaving the vessel is containing water and the balance is acetic acid. The hexanolrich extract is fed to a distillation column. The composition of the distillate is acetic acid and the balance is hexanol. The bottom stream contains hexanol and recovers of the hexanol fed to the liquidliquid extraction vessel.
a Draw and completely label the process flowchart.
Marks
b Perform degree of freedom analysis on each unit process and overall system.
Marks
c If the amount of pure hexanol fed to the extraction vessel is calculate
i the flowrate for each stream,
ii the composition of hexanolrich stream, and
iii percentage of acetic acid in the process that is recovered in the distillate stream.
Marks
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


