Question: In a two stage process, acetic acid (A) is extracted from water (W) into hexanol (H) in a liquid- liquid extraction vessel and the extract
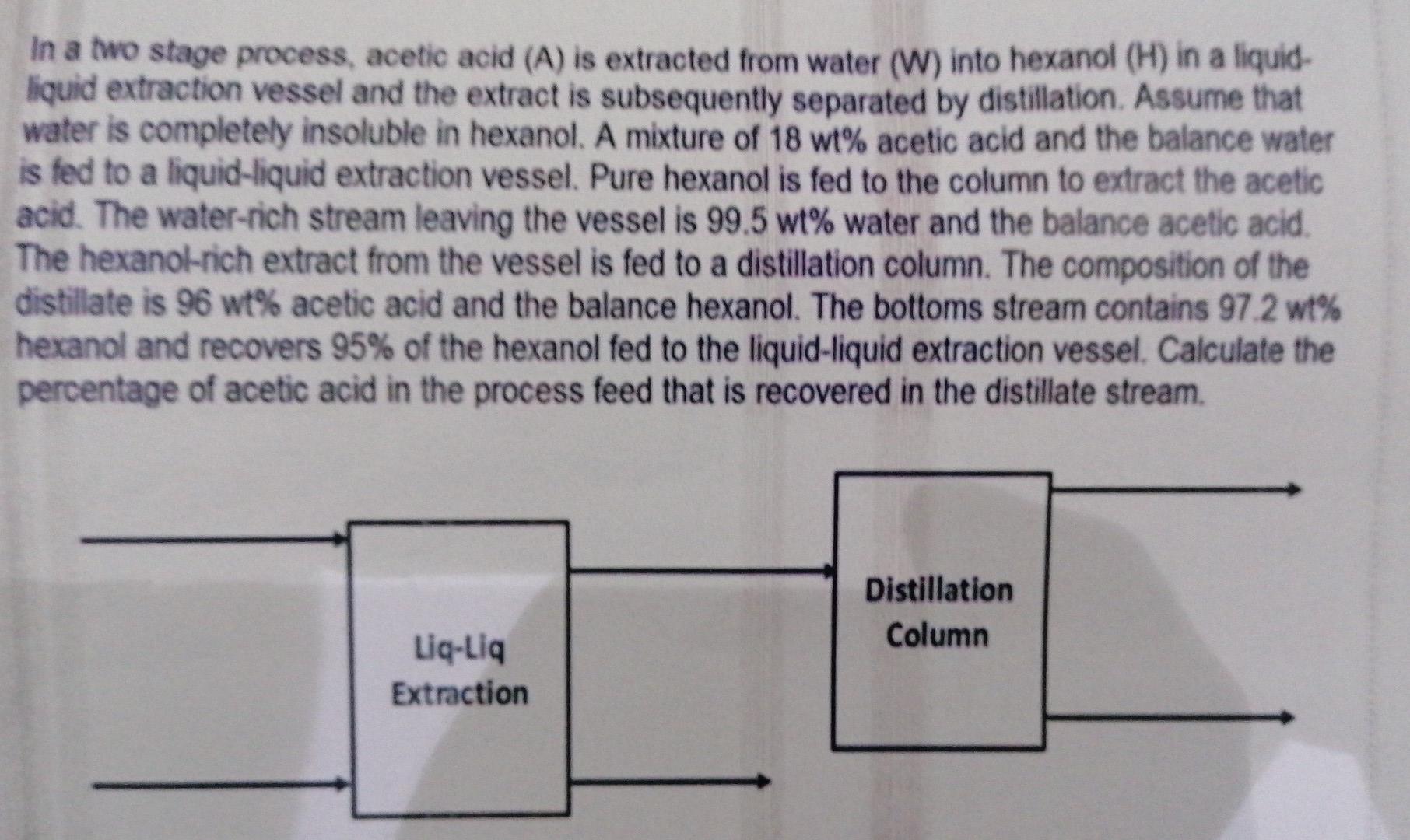
In a two stage process, acetic acid (A) is extracted from water (W) into hexanol (H) in a liquid- liquid extraction vessel and the extract is subsequently separated by distillation. Assume that water is completely insoluble in hexanol. A mixture of 18 wt% acetic acid and the balance water is fed to a liquid-liquid extraction vessel. Pure hexanol is fed to the column to extract the acetic acid. The water-rich stream leaving the vessel is 99.5 wt% water and the balance acetic acid The hexanol-rich extract from the vessel is fed to a distillation column. The composition of the distillate is 96 wt% acetic acid and the balance hexanol. The bottoms stream contains 97.2 wt% hexanol and recovers 95% of the hexanol fed to the liquid-liquid extraction vessel. Calculate the percentage of acetic acid in the process feed that is recovered in the distillate stream Distillation Column Liq-Liq Extraction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


