Question: In system A ( PFR followed by CSTR ) , what is the conversion in the first PFR ? % In system A ( PFR
In system A PFR followed by CSTR what is the conversion in the first PFR
In system A PFR followed by CSTR what is finaloverall conversion? State which system will give the highest overall conversion?
System A PFR followed by CSTR
System B CSTR followed by PFR
System C parallelYou have reactors of equal volume liters available for your use: one a CSTR and the other a PFR
The reaction is irreversible, isothermal and follows secondorder kinetics.
The rate constant,
The initial reactant concentration, is liter.
The volumetric flow rate is litersh
There are ways you can arrange the system:
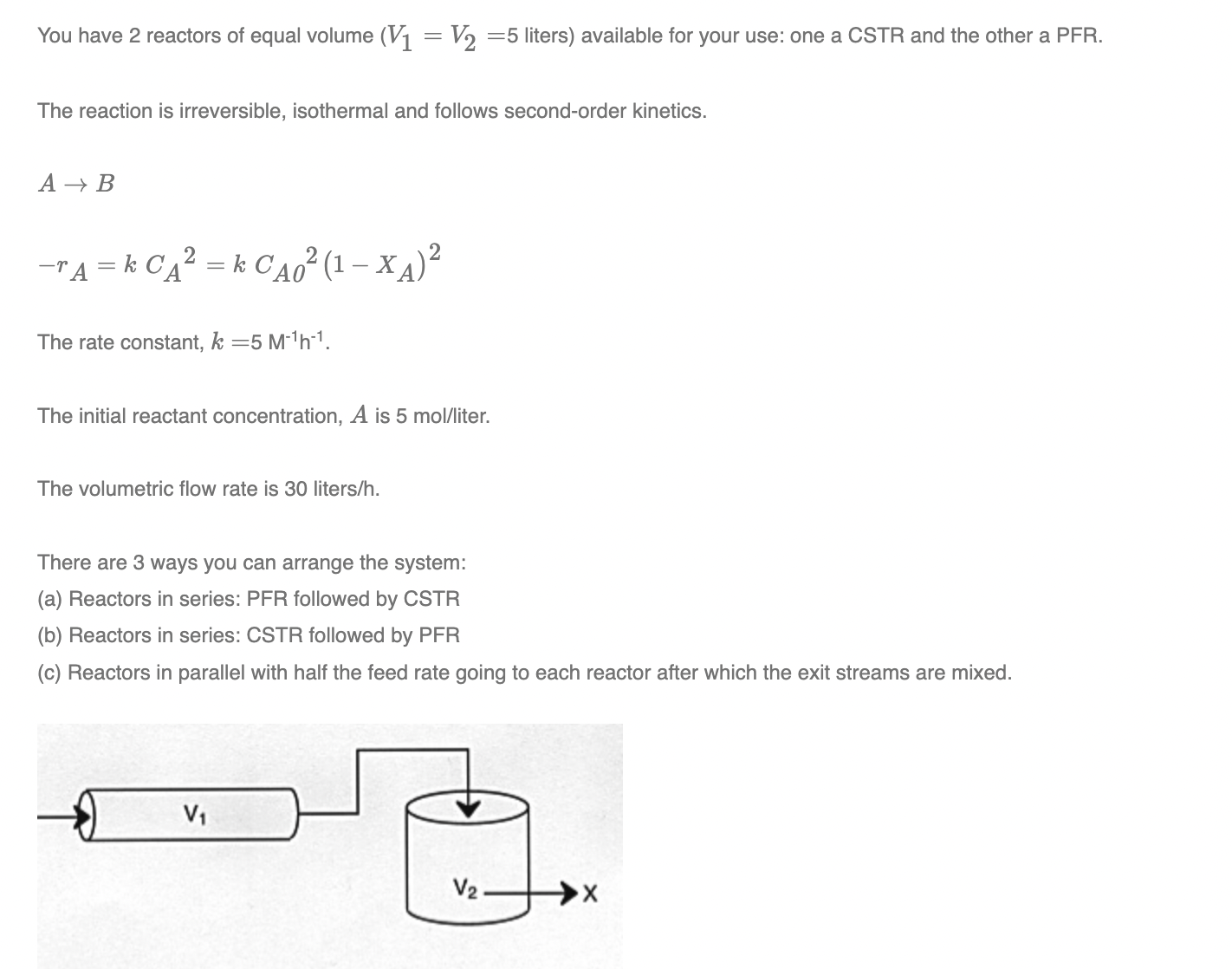
a Reactors in series: PFR followed by CSTR
b Reactors in series: CSTR followed by PFR
c Reactors in parallel with half the feed rate going to each reactor after which the exit streams are mixed.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


