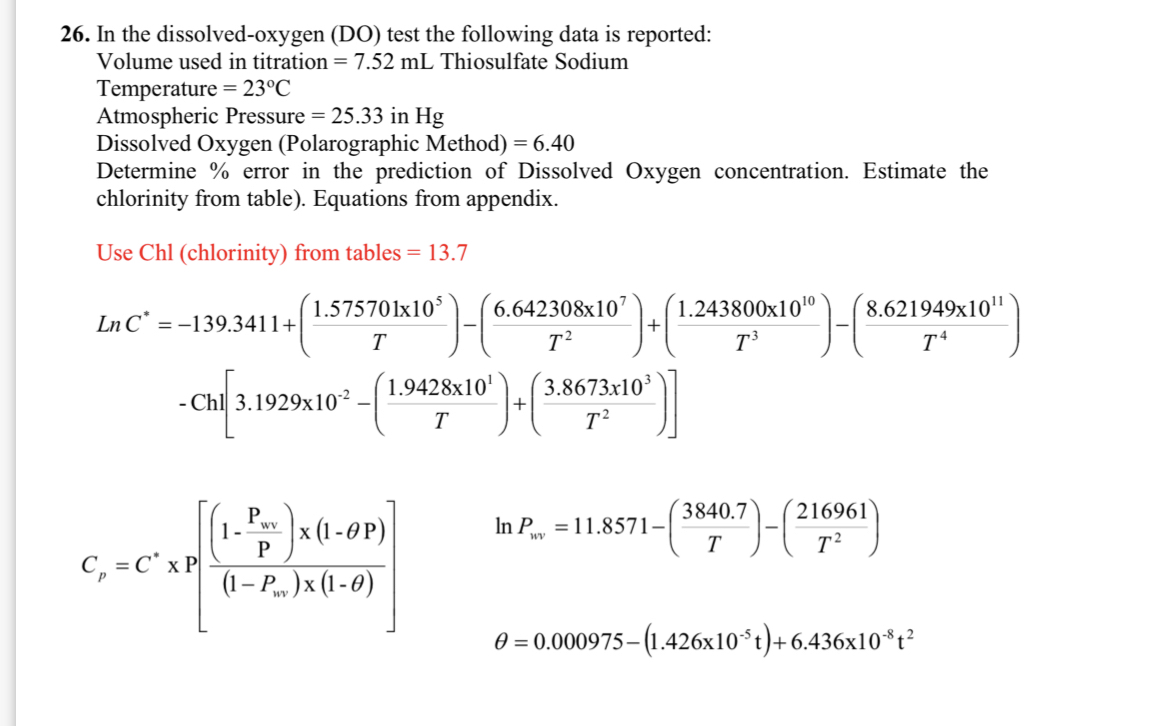
Question: In the dissolved - oxygen ( DO ) test the following data is reported: Volume used in titration = 7 . 5 2 m L
In the dissolvedoxygen DO test the following data is reported:
Volume used in titration Thiosulfate Sodium
Temperature
Atmospheric Pressure in
Dissolved Oxygen Polarographic Method
Determine error in the prediction of Dissolved Oxygen concentration. Estimate the chlorinity from table Equations from appendix.
Use Chl chlorinity from tables
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


