Question: In the process sketched below, Na 2 CO 3 is produced by the reaction: The reaction is 90 % complete on one pass through the
In the process sketched below, Na2CO3 is produced by the reaction:
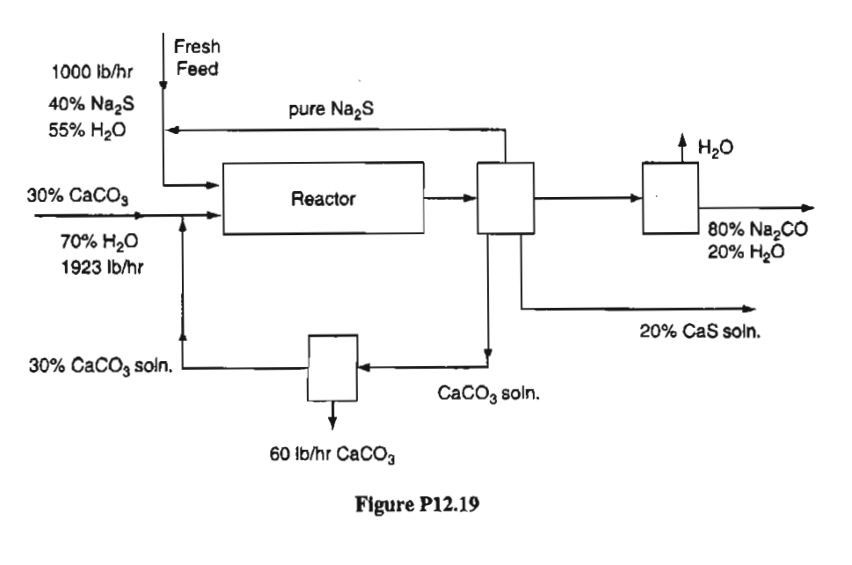
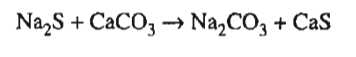 The reaction is 90 % complete on one pass through the reactor and the amount of CaCO3 entering the reactor is 50 % in excess of that needed. Calculate on the basis of 1 000 lb/hr of fresh feed:
The reaction is 90 % complete on one pass through the reactor and the amount of CaCO3 entering the reactor is 50 % in excess of that needed. Calculate on the basis of 1 000 lb/hr of fresh feed:
a) the lb of Na2S recycled b) the lb of Na2CO3 solution formed per hour
Na2S+CaCO3Na2CO3+CaS =G2n=m=man Na2S+CaCO3Na2CO3+CaS =G2n=m=man
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


