Question: In this project each group is going to study the reaction in a continuous stirred-tank reactor (CSTR). As you have seen in the course, CSTR's

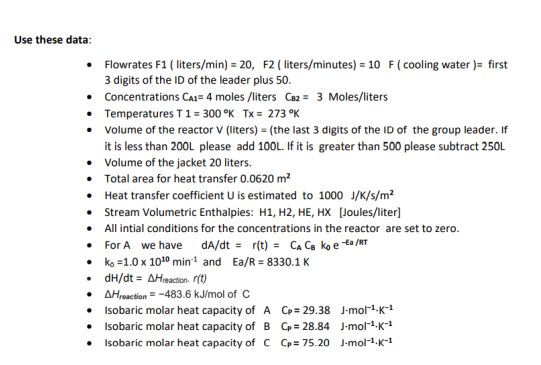
In this project each group is going to study the reaction in a continuous stirred-tank reactor (CSTR). As you have seen in the course, CSTR's can operate continuously and provide several advantages. The CSTR in this project is jacketed, none adiabatic and it is not isothermal. A schematic of our process is shown in the figure in bellow. Reaction in the reactor For A we have A+BC with a rate dA/dt=r(t)=CACBk0eEa/ATk0=1.01010min1andEa/R=8330.1KdH/dt=Hreaction.r(t)Hresecion=483.6kJ/molofC Isobaric molar heat capacity of A CP=29.38Jmol1K1 Isobaric molar heat capacity of BCP=28.84Jmol1K1 Isobaric molar heat capacity of DCP=75.20Jmol1K1 Steady-state analysis: - Show how steady states are solutions to a system of nonlinear algebraic equations. - Solve a system of nonlinear algebraic equations using MATLAB Unsteady-state analysis Apply a step increase in CA1 of a magnitude of 0.2mole/m3 and keep C82 unchanged Use the information in the lectures to demonstrate how the transient behavior of the reactor is a solution to a system of nonlinear ordinary differential equations. - Solve a system of nonlinear ordinary differential equations using finite differences. - Write and run a MATLAB to find the solution. se these data: - Flowrates F1 ( liters /min)=20,F2 ( liters / minutes )=10F ( cooling water )= first 3 digits of the ID of the leader plus 50 . - Concentrations CAA=4 moles /liters C82=3 Moles/liters - Temperatures T=300KTx=273K - Volume of the reactor V (liters) = (the last 3 digits of the ID of the group leader. If it is less than 200L please add 100L. If it is greater than 500 please subtract 250L - Volume of the jacket 20 liters. - Total area for heat transfer 0.0620m2 - Heat transfer coefficient U is estimated to 1000J/K/s/m2 - Stream Volumetric Enthalpies: H1, H2, HE, HX [Joules/liter] - All intial conditions for the concentrations in the reactor are set to zero. - For A we have dA/dt=r(t)=CACBk0eta/RT - k0=1.01010min1 and Ea/R=8330.1K - dH/dt=Hreaction.r(t) - Hresetion=483.6kJ/mol of C - Isobaric molar heat capacity of A CP=29.38Jmol1K1 - Isobaric molar heat capacity of B Cp=28.84Jmol1K1 - Isobaric molar heat capacity of CCp=75.20Jmol1K1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


