Question: In which one of the following reactions will a decrease in total pressure at constant temperature favour formation of reactants? Select one: 0 a. H,(g)
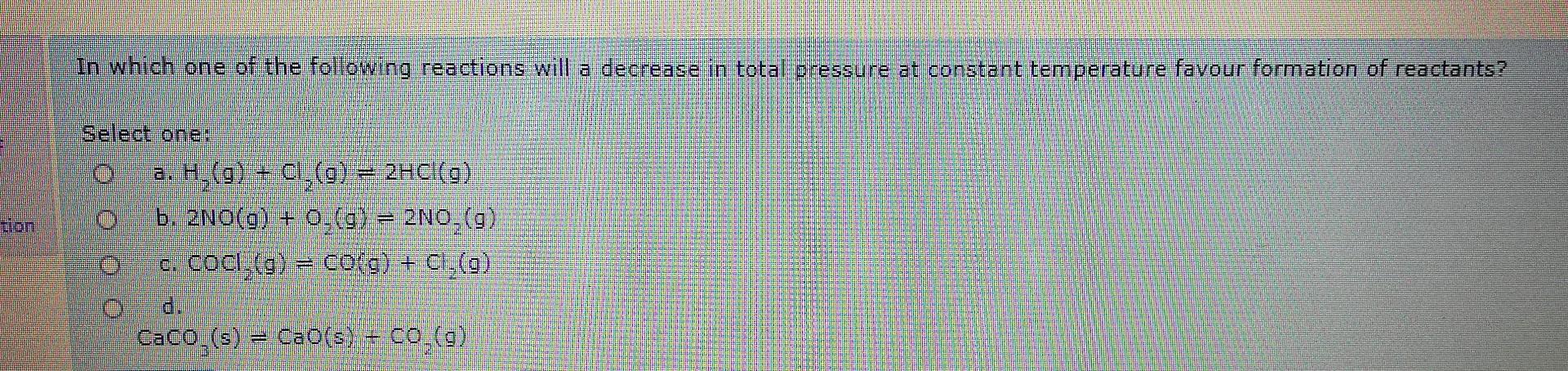
In which one of the following reactions will a decrease in total pressure at constant temperature favour formation of reactants? Select one: 0 a. H,(g) + Cl (9) = 2HCl(9) b. 2NO(g) + 0.(9) = 2NO,(g) c. cocl (g) = co(g) + CH-(0) Caco-(s) CD(= CCB
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


