Question: Indicate whether each statement about atomic energy levels is true or false. 1. The atom shown in the diagram has four energy levels or shells.
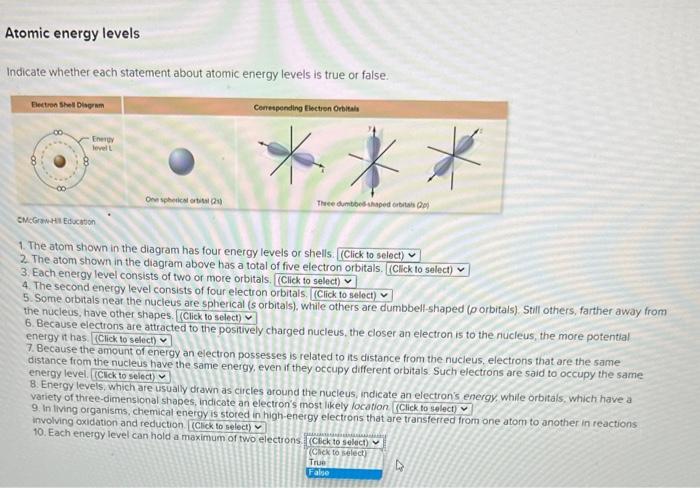
Indicate whether each statement about atomic energy levels is true or false. 1. The atom shown in the diagram has four energy levels or shells. 2. The atom shown in the diagram above has a total of five electron orbitals. 3. Each energy level consists of two or more orbitals. 4. The second energy level consists of four electron orbitals. 5. Some orbitals near the nucleus are sphenical (s orbitals), while others are dumbbell-shaped ( p orbitals). Still others, farther away from the nucleus, have other shapes. 6. Because electrons are attracted to the positively charged nucleus, the closer an electron is to the nucleus, the more potential energy it has. 7. Because the amount of energy an electron possesses is related to its distance from the nucleus, electrons that are the same distance from the nucleus have the same energy, even if they occupy different orbitals. Such electrons are said to occupy the same energy level. 8. Energy levens, which are usually drawn as circles around the nucleus, indicate an electron's energy, while orbitals, which have a variety of three-dimensional shapes, indicate an electron's most likely location 9. In tiving organisms, chemical enerovis stored in high-energy electrons that are transferred from one atom to another in reactions. involving oxidation and reduction. 10. Each energy level can hold a maximum or two electrons
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


