Question: INFORMATION: AG = AH TAS = -RT In K R= 8.314 J/mol.K For the reaction aA + bB CC + dD, AH' = CAH/(C)+ DAH;
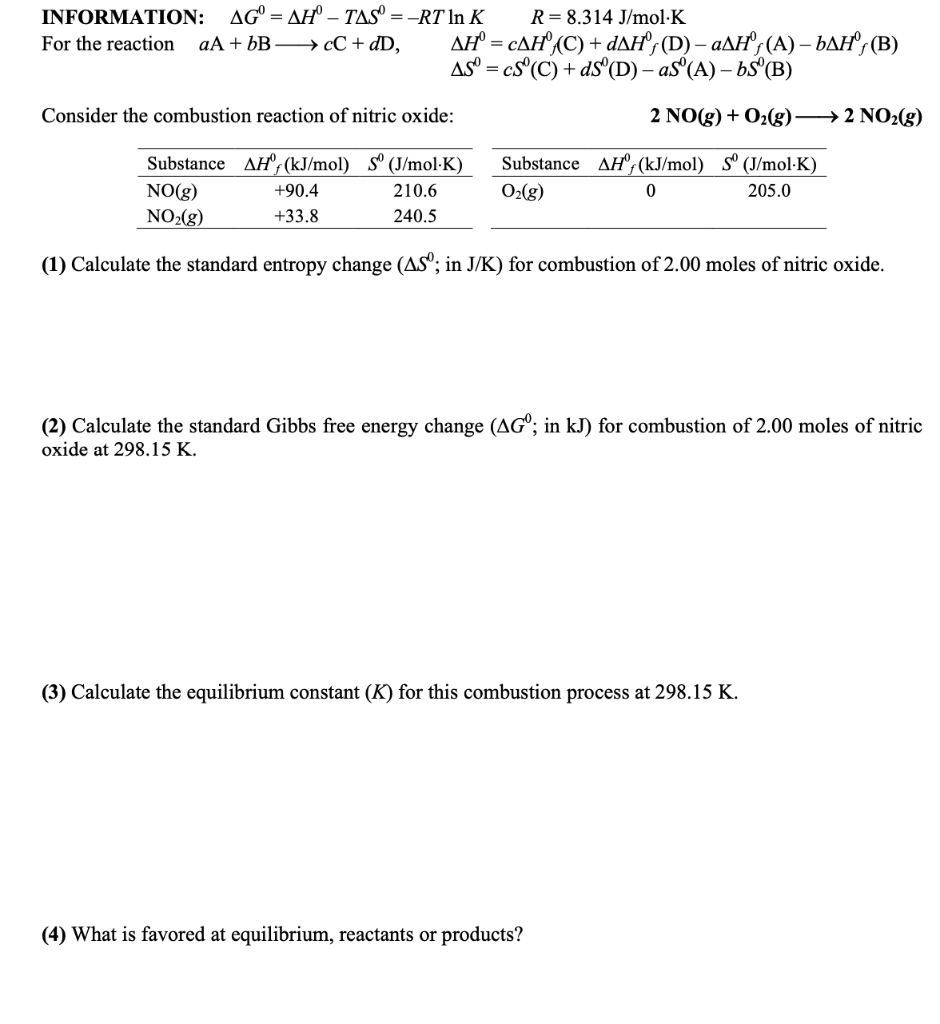
INFORMATION: AG = AH TAS = -RT In K R= 8.314 J/mol.K For the reaction aA + bB CC + dD, AH' = CAH/(C)+ DAH; (D) ZAH;(A) BAH/(B) AS = cs(C) + d3(D) aS"(A) 65(B) - = Consider the combustion reaction of nitric oxide: 2 NO(g) + O2(g) 2 NO2(g) Substance AH-(kJ/mol) 5 (J/mol K) NO(g) +90.4 210.6 NO2(g) +33.8 240.5 Substance AH'/(kJ/mol) S (J/mol K) O2(g) 0 205.0 (1) Calculate the standard entropy change (AS; in J/K) for combustion of 2.00 moles of nitric oxide. (2) Calculate the standard Gibbs free energy change (AG; in kJ) for combustion of 2.00 moles of nitric oxide at 298.15 K. (3) Calculate the equilibrium constant (K) for this combustion process at 298.15 K. (4) What is favored at equilibrium, reactants or products
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


