Question: Internal energy (U) is a function of temperature and volume and is expressed by the following 2 na differential equation: du = nCvdT +
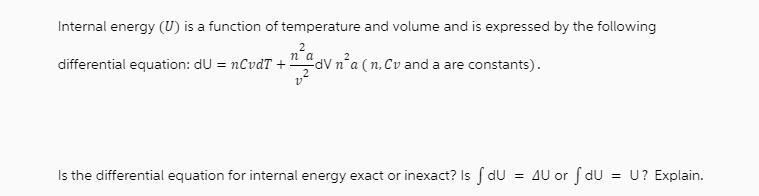
Internal energy (U) is a function of temperature and volume and is expressed by the following 2 na differential equation: du = nCvdT + "dV n'a (n. Cv and a are constants). Vn Is the differential equation for internal energy exact or inexact? Is f du = 4U or f du = U? Explain.
Step by Step Solution
3.51 Rating (158 Votes )
There are 3 Steps involved in it
Solution The differential equation for internal energy in the image is exact This means that the int... View full answer

Get step-by-step solutions from verified subject matter experts


