Question: iodine clock reaction please show in excel and working create two graphs showing how you obtained the value for m or n so that you
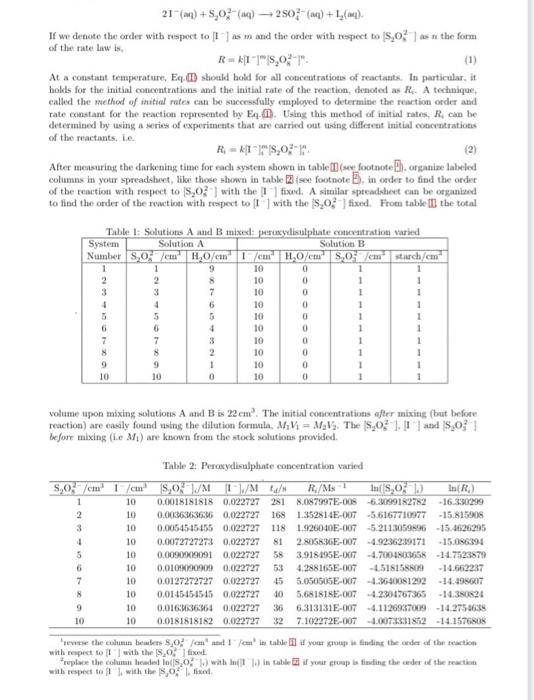
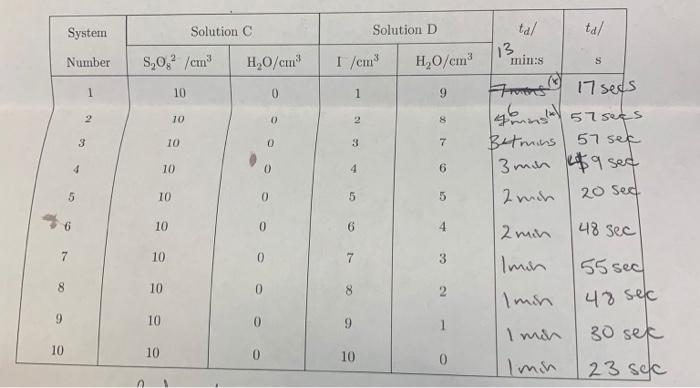
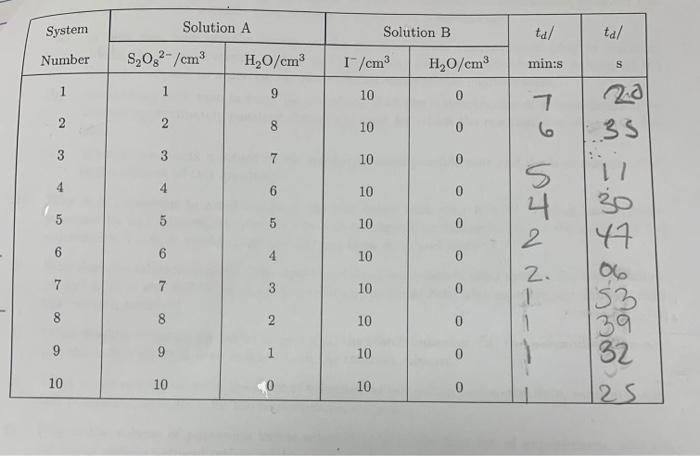
21(aq)+S2O82(aq)2SO42(aq)+I2(aq) If we denote the order with respect to [I-] as m and the order with respect to [S2O42 ] as n the form of the rate law is, R=k[I]m[S2O42]n. At a constant temperature, Eq- ED should hold for all concentrations of reactants. In particular, it holds for the initial concentrations and the initial rate of the reaction, denoted as Ri.. A technique, called the method of initial rates can be saccessfully employed to determine the reaction order and rate constant for the reaction represented by Eq- i.). Using this method of initial nates, R4 can be determined by using a series of experiments that are carried out using diffetent initial concentrations of the reactants, i.e. R1=k1]im/S2On2]in. After measuring the darkening time for each system shown in table (B) (see footnote D), organize labeled columns in your spreadshect, like those shown in table (see footnote E ), in order to find the order of the reaction with respect to [S2O52] with the I]fixed. A similar spteadshert can be ofganiand to find the order of the reaction with respect to [ ] ] with the [S2O52] fixed. Frota table III the total volume upon mixing solutions A and B is 22cm3. The initial concentrations ofter mixing (but before reaction) are easily found using the dilution formula, M1V1=M2V2. The {S2O52]. [1 and S2O32] before mixing (i.e M1 ) are known frotn the stock solutions provided. Table 2: Peroxydisulphate concentration varied with repeet to [I1] with the [B2O32] firoul. with respect to [I I, with the S3O422 l. fixed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


