Question: is anyone able to explain with steps? thank u much apprecited A 4.50104mol sample of Ca(OH)2 requires 10.00mL of HCl(aq) for neutralization according to the
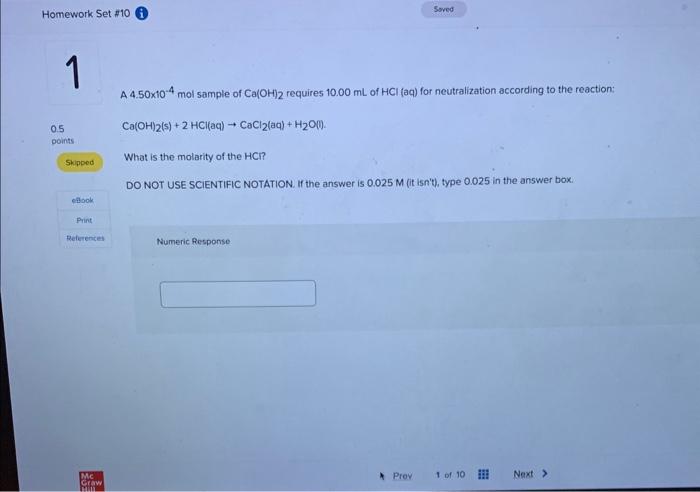
A 4.50104mol sample of Ca(OH)2 requires 10.00mL of HCl(aq) for neutralization according to the reaction: Ca(OH)2(s)+2HCl(aq)CaCl2(aq)+H2O(l) What is the molarity of the HCl ? DO NOT USE SCIENTIFIC NOTATION. If the answer is 0.025M (it isn't), type 0.025 in the answer box. Numeric Response
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


