Question: Isotope Y ( a short lived nuclide ) and Z ( a longer lived nuclide ) are mixed in a sample,
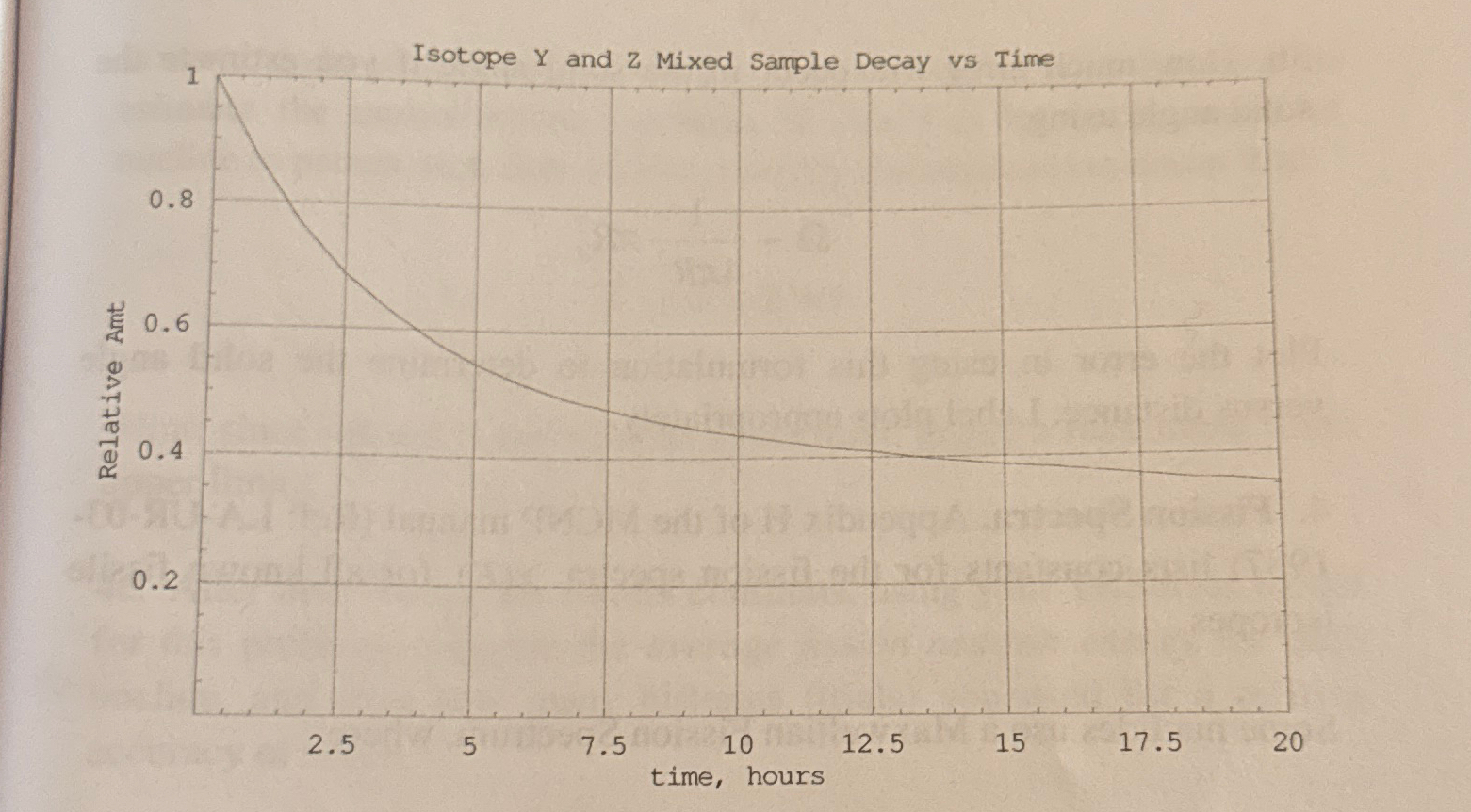
Isotope Ya short lived nuclide and Za longer lived nuclide are mixed in a sample, and each has two very different half lives. The plot below gives the fraction of isotopes Y and Z remaining in the mixed sample versus time in hours
Estimate the half lifes of isotopes Y and Z using the mixture plot. State untits.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


