Question: It is proposed to separate CaCO3 (solid) from slurry via crystallization. The feed slurry contains equal mass fractions of CaCO3 precipitate in a solution of
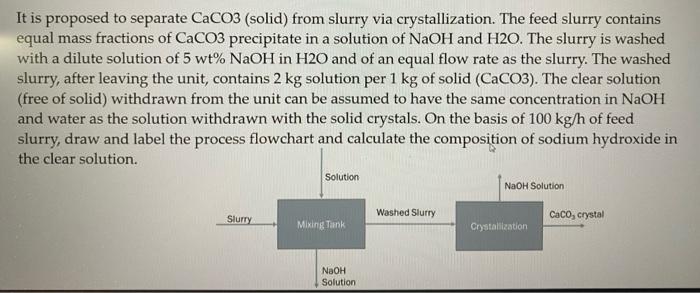
It is proposed to separate CaCO3 (solid) from slurry via crystallization. The feed slurry contains equal mass fractions of CaCO3 precipitate in a solution of NaOH and H2O. The slurry is washed with a dilute solution of 5 wt% NaOH in H20 and of an equal flow rate as the slurry. The washed slurry, after leaving the unit, contains 2 kg solution per 1 kg of solid (CaCO3). The clear solution (free of solid) withdrawn from the unit can be assumed to have the same concentration in NaOH and water as the solution withdrawn with the solid crystals. On the basis of 100 kg/h of feed slurry, draw and label the process flowchart and calculate the composition of sodium hydroxide in the clear solution. Solution NaOH Solution Washed Slurry Slurry Caco, crystal Mixing Tank Crystallization NaOH Solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


