Question: It is typically very difficult to do a substitution reaction on an aromatic ring when the leaving group is flanked by two other bulky substituents.
It is typically very difficult to do a substitution reaction on an aromatic ring when the leaving group is flanked by two other bulky substituents. Moreover, in Section 22.3, we found that nucleophilic aromatic substitution requires strongly electron-withdrawing groups on the benzene ring. However, Pd-catalyzed coupling allows entry into such products. As examples, write the products of the following reactions and state which coupling reaction is being utilized.
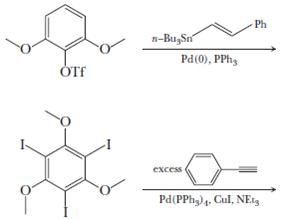
OTF n-BugSn Pd(0), PPh excess Ph Pd(PPhg) 4, Cul, NEts
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


