Question: Its the Carboxylic Acid-Phenol-Neutral Separation by Acid-Base Extraction lab. Organic layer Neutral compound Organic layer Phenol/neutral compounds Evaporate solvent Neutral Compound Acid/Base Extraction Flow Chart
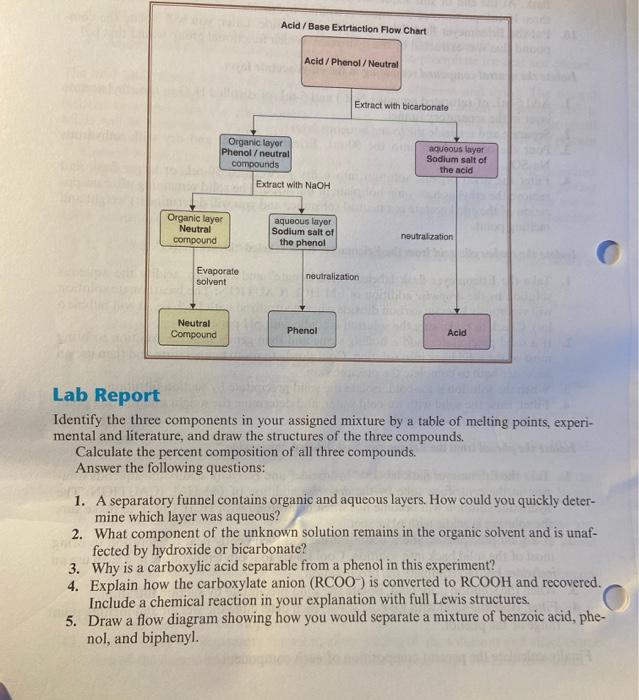

Organic layer Neutral compound Organic layer Phenol/neutral compounds Evaporate solvent Neutral Compound Acid/Base Extraction Flow Chart Acid/Phenol / Neutral Extract with NaOH aqueous layer Sodium salt of the phenol Extract with bicarbonate neutralization Phenol aqueous layer Sodium salt of the acid neutralization Acid Lab Report Identify the three components in your assigned mixture by a table of melting points, experi- mental and literature, and draw the structures of the three compounds. Calculate the percent composition of all three compounds. Answer the following questions: 1. A separatory funnel contains organic and aqueous layers. How could you quickly deter- mine which layer was aqueous? 2. What component of the unknown solution remains in the organic solvent and is unaf- fected by hydroxide or bicarbonate? 3. Why is a carboxylic acid separable from a phenol in this experiment? 4. Explain how the carboxylate anion (RCOO) is converted to RCOOH and recovered. Include a chemical reaction in your explanation with full Lewis structures. 5. Draw a flow diagram showing how you would separate a mixture of benzoic acid, phe- nol, and biphenyl.
Step by Step Solution
3.39 Rating (146 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


