Question: Jsing properties listed in the table below from a vendor, estimate the enthalpy of sublimation and ihe entropy of sublimation of CO2. a) Sketch a

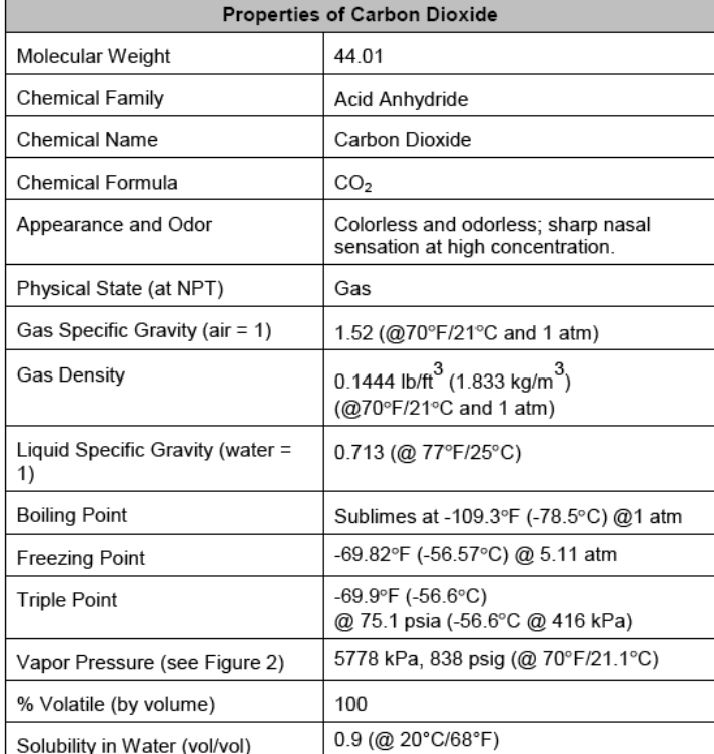
Jsing properties listed in the table below from a vendor, estimate the enthalpy of sublimation and ihe entropy of sublimation of CO2. a) Sketch a CO2(p,T) phase diagram; label areas in the diagram as solid, liquid or gas; and indicate ihe data points that you used for the calculation. b) Explain why the Clausius-Clapeyron equation can be applied in this case. c) Compare your calculated values with reported values. Properties of Carbon Dioxide \begin{tabular}{|l|l|} \hline Molecular Weight & 44.01 \\ \hline Chemical Family & Acid Anhydride \\ \hline Chemical Name & Carbon Dioxide \\ \hline Chemical Formula & CO2 \\ \hline Appearance and Odor & Colorlessandodorless;sharpnasalsensationathighconcentration. \\ \hline Physical State (at NPT) & Gas \\ \hline Gas Specific Gravity (air =1) & 1.52(@70F/21C and 1atm) \\ \hline Gas Density & 0.1444lb/ft3(1.833kg/m3)(@70F/21Cand1atm) \\ \hline LiquidSpecificGravity(water=1) & 0.713(@77F/25C) \\ \hline Boiling Point & Sublimes at 109.3F(78.5C)@1atm \\ \hline Freezing Point & 69.82F(56.57C)@5.11atm \\ \hline Triple Point & 69.9F(56.6C) \\ @ 75.1psia(56.6C@416kPa) \\ \hline Vapor Pressure (see Figure 2) & 5778kPa,838psig(@70F/21.1C) \\ \hline \% Volatile (by volume) & 100 \\ \hline Solubility in Water (vol/vol) & 0.9(@20C/68F) \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


