Question: just need help setting up for the first C metal equation to do all of them! thank you!! cmetal=mmetal(TboilTfinal)(mcalccal+mwatercwater)(TfinalTstart) begin{tabular}{|c|c|} hline Metal & Specific Heat
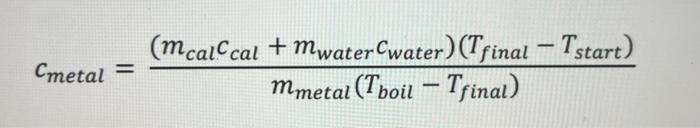
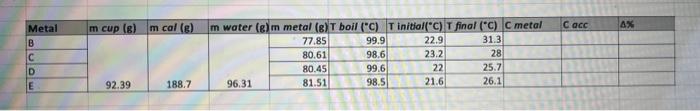
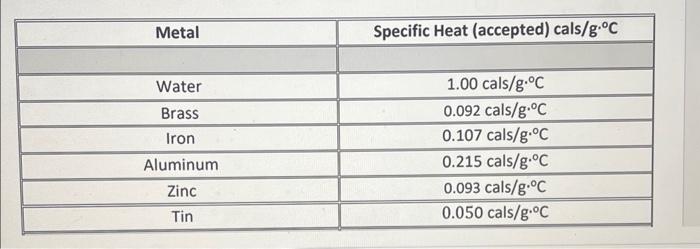
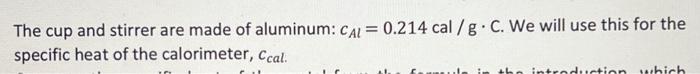
cmetal=mmetal(TboilTfinal)(mcalccal+mwatercwater)(TfinalTstart) \begin{tabular}{|c|c|} \hline Metal & Specific Heat (accepted) cals/g. C \\ \hline Water & 1.00cals/gC \\ \hline Brass & 0.092cals/gC \\ \hline Iron & 0.107cals/gC \\ \hline Aluminum & 0.215cals/gC \\ \hline Zinc & 0.093cals/gC \\ \hline Tin & 0.050cals/gC \\ \hline \hline \end{tabular} The cup and stirrer are made of aluminum: cAl=0.214cal/gC. We will use this for the specific heat of the calorimeter, ccal
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


