Question: just the answer no explanation needed 8. A 0.50 M solution of a weak base B has a pH of 10.67. The equation for ionization
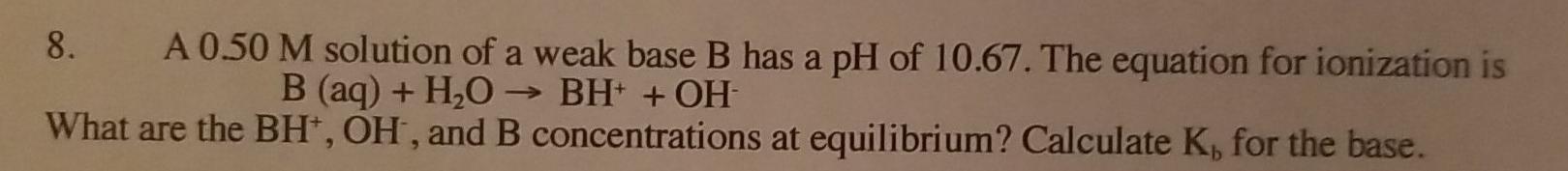
just the answer no explanation needed
8. A 0.50 M solution of a weak base B has a pH of 10.67. The equation for ionization is B (aq) + H2O BH+ + OH What are the BH, OH, and B concentrations at equilibrium? Calculate K for the base
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


