Question: just the answer no explanation needed 9. The base hydrazine, NH4, has a pK, equal to 5.90. Calculate the hydroxide concentration, the pH. and the
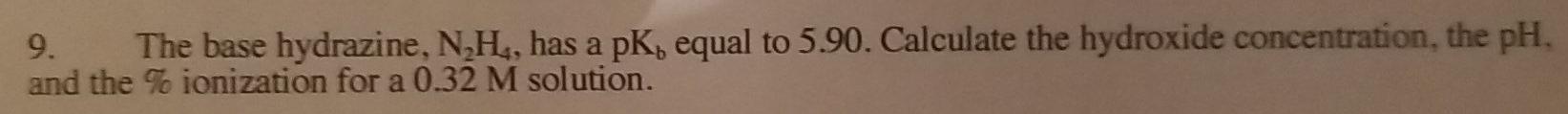
just the answer no explanation needed
9. The base hydrazine, NH4, has a pK, equal to 5.90. Calculate the hydroxide concentration, the pH. and the T ionization for a 0.32 M solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


