Question: Problem 16. (1 point) Which of the following is the most likely formula for a compound of Cs (atomic number = 55) and Se
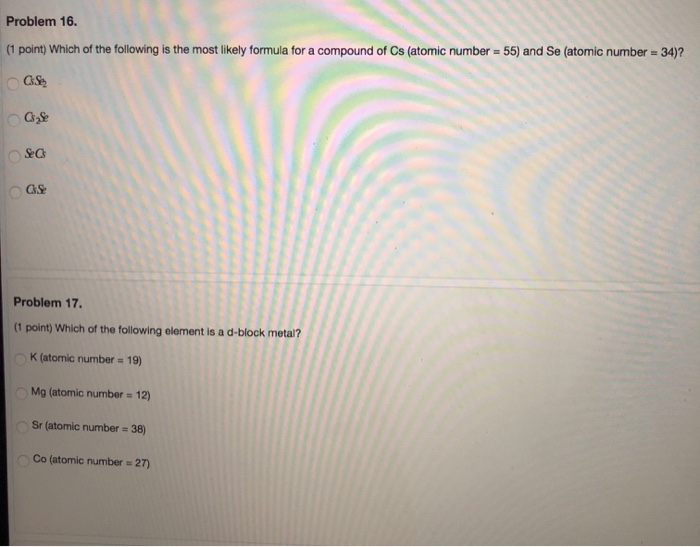
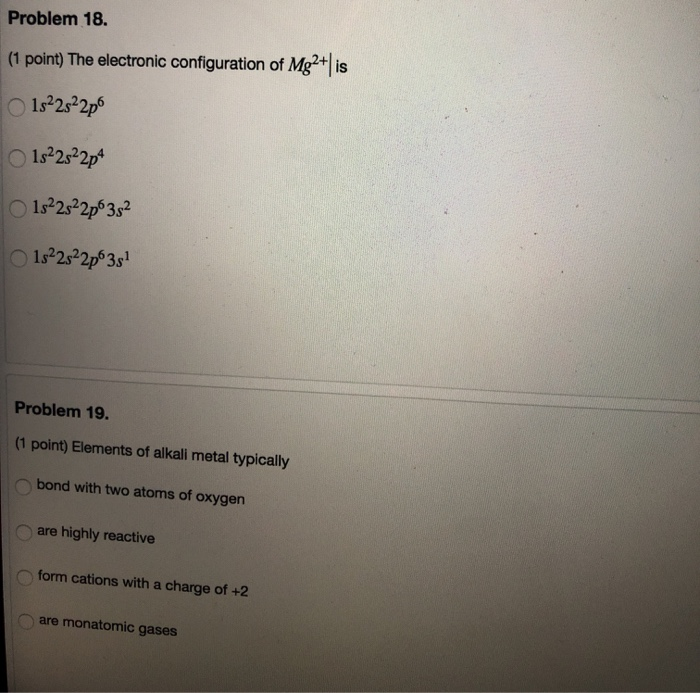
Problem 16. (1 point) Which of the following is the most likely formula for a compound of Cs (atomic number = 55) and Se (atomic number = 34)? OGS C Se Cs OGS Problem 17. (1 point) Which of the following element is a d-block metal? K (atomic number = 19) Mg (atomic number = 12) Sr (atomic number = 38) Co (atomic number = 27) Problem 18. (1 point) The electronic configuration of Mg2+ | is 1s2s2p6 1s2s22p4 1s2s2p6 35 1s2s2p63s Problem 19. (1 point) Elements of alkali metal typically bond with two atoms of oxygen O are highly reactive form cations with a charge of +2 are monatomic gases
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
The detailed answer for the above question is provided below Problem 16 Using a periodic table we ca... View full answer

Get step-by-step solutions from verified subject matter experts


