Question: Just the last question please: Your Answer Correct Answer Correct From balances on the overall system and the reaction stoichiometry calculate the following quantities. Molar
Just the last question please:
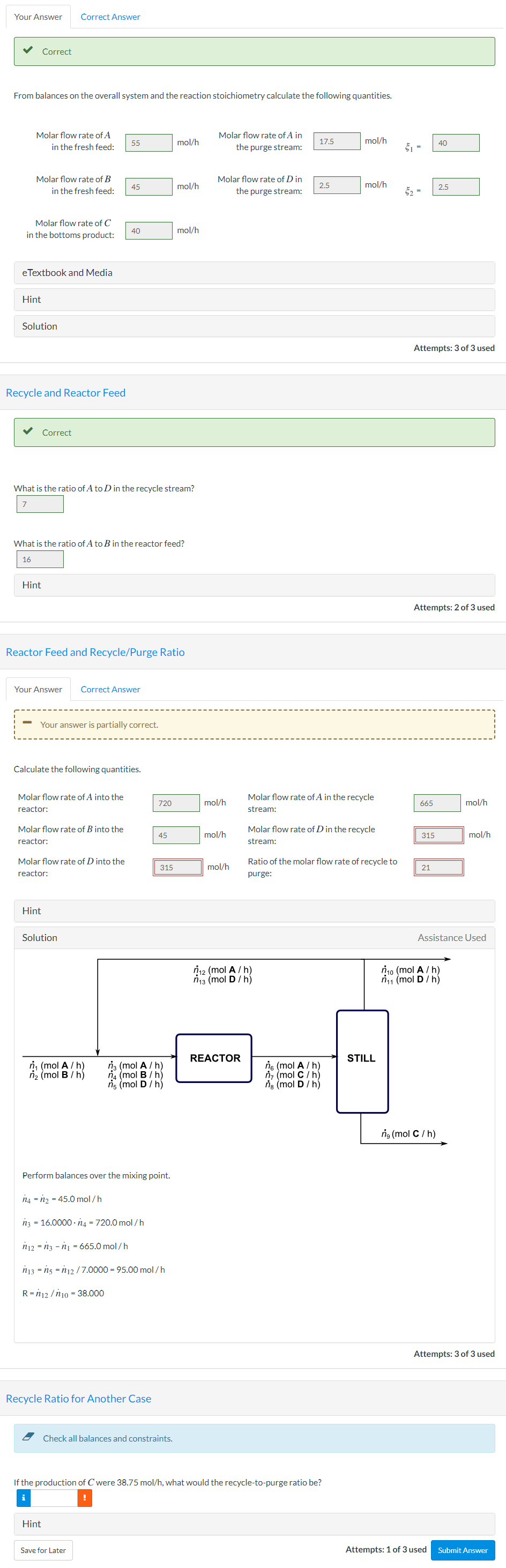
Your Answer Correct Answer Correct From balances on the overall system and the reaction stoichiometry calculate the following quantities. Molar flow rate of A in the fresh feed: 55 mol/h Molar flow rate of A in the purge stream: 17.5 mol/h 40 61 Molar flow rate of B in the fresh feed: 45 mol/h Molar flow rate of Din the purge stream: 2.5 mol/h 2.5 62 Molar flow rate of C in the bottoms product: 40 mol/h e Textbook and Media Hint Solution Attempts: 3 of 3 used Recycle and Reactor Feed Correct What is the ratio of A to D in the recycle stream? What is the ratio of A to B in the reactor feed? 16 Hint Attempts: 2 of 3 used Reactor Feed and Recycle/Purge Ratio Your Answer Correct Answer Your answer is partially correct. Calculate the following quantities. Molar flow rate of A into the reactor: 720 mol/h Molar flow rate of A in the recycle stream: 665 mol/h Molar flow rate of B into the reactor: 45 mol/h Molar flow rate of D in the recycle stream: 315 mol/h Molar flow rate of D into the reactor: 315 mol/h Ratio of the molar flow rate of recycle to purge: 21 Hint Solution Assistance Used n 12 (mol A/h) n 13 (mol D /h) n 10 (mol A/h) nu (mol D /h) REACTOR STILL n (mol A/h) n (mol B/h) na (mol A/h) na (mol B/h) ns (mol D / h) ng (mol A/ h) n (mol C/h) ng (mol D/ h) ng (mol C / h) Perform balances over the mixing point. 14 = n2 = 45.0 mol/h n3 = 16.0000.14 = 720.0 mol/h n12 = n3 - n1 = 665.0 mol/h n3 = n3 = 112 /7.0000 = 95.00 mol/h R=112 / n 10 = 38.000 Attempts: 3 of 3 used Recycle Ratio for Another Case Check all balances and constraints. If the production of C were 38.75 mol/h, what would the recycle-to-purge ratio be? ! Hint Save for Later Attempts: 1 of 3 used Submit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


