Question: Kinetics Exercises 1. Consider the reaction 2A+BC+2D which has a rate expression of rate =k[A][B]2 Answer the following questions as specifically as possible. a) What
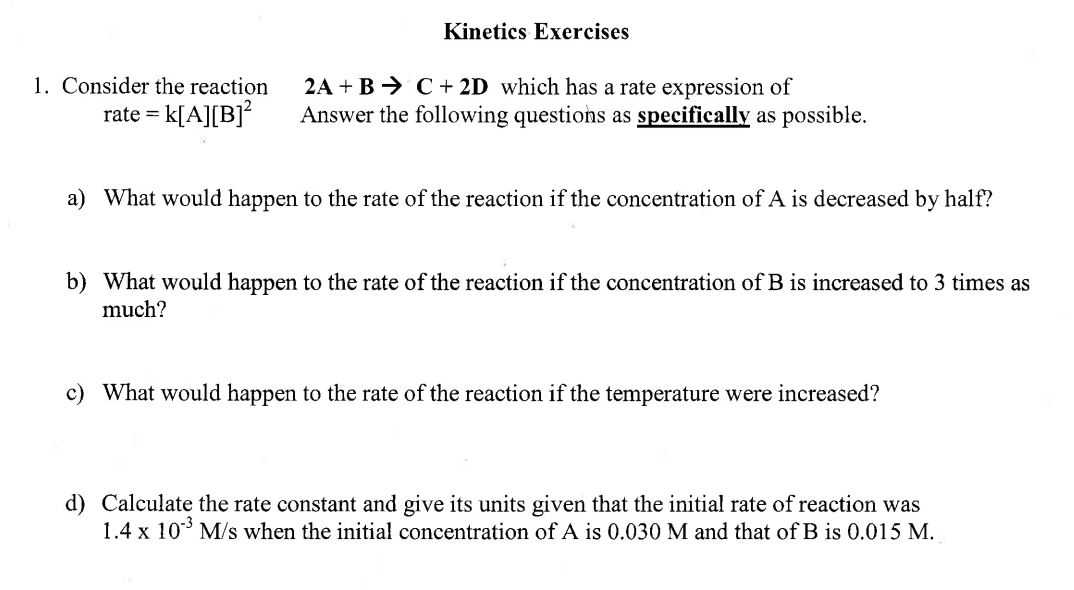
Kinetics Exercises 1. Consider the reaction 2A+BC+2D which has a rate expression of rate =k[A][B]2 Answer the following questions as specifically as possible. a) What would happen to the rate of the reaction if the concentration of A is decreased by half? b) What would happen to the rate of the reaction if the concentration of B is increased to 3 times as much? c) What would happen to the rate of the reaction if the temperature were increased? d) Calculate the rate constant and give its units given that the initial rate of reaction was 1.4103M/s when the initial concentration of A is 0.030M and that of B is 0.015M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


