Question: Lab 17 - Design models (simulation software or 3 D model) to compare structural and geometric isomers. BACKGROUND Hydrocarbons Hydrocarbons are organic compounds that consist

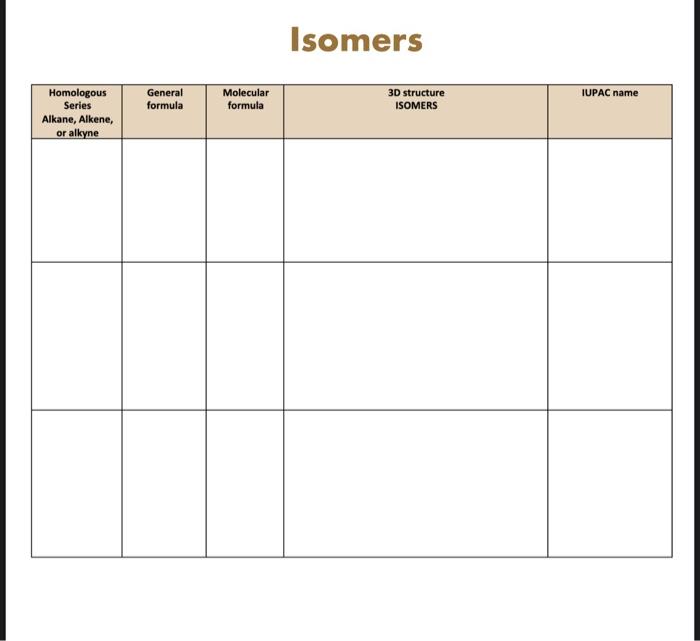
Lab 17 - Design models (simulation software or 3 D model) to compare structural and geometric isomers. BACKGROUND Hydrocarbons Hydrocarbons are organic compounds that consist primarlly of carbon and hydrogen atoms. They are vital building blocks for the creation of many useful products, including fuels, plastics, and pharmaceuticals. Hydrocarbons can be categorized into two main types-aliphatic and aromatic-based of their structure and bonding. Aliphatic hydrocarbons are chains or branched chains of carbon atoms, while aromatic mydrocarbons contain one or mere rings of carbon atoms. Hydrocarbans can be obtained from natural sources such as crude oil, natural gas, and coal, or they can be synthesized in the laboratory using various methods. Some hydrecarbons are considered harmful pollutants and can contribute to ait pollution and climate change when released into the atmosphere. Proper safety precautions should be taken when handling and working with hydrocarbons to avoid accidents and minimize environmental damage. Isomers Hydrecarbons are organic compounds compesed of hydrogen and carbon atoms. isemers of hydrecarbons are compeunds that have the same molecular formula but different structural formulas. meaning the atoms are arranged in a different way. There are two main types of isomers of hydrocarbons - structural isomers and geometric isomers. Structural isomers have different bonding patterns between their carbon atoms, creating different structural formulas. For example, butane and isobutane are structural isomers because they have the same molecular formula but different structural formulas. Geometric isemers have different arrangements of their atoms with respect to a double bond or ring structure. Hence, they have different physical and chemical properties. For example, cis-2-butene and trans-2-butene are geometric isemers. On the basis on your knowledge to Hydrocarbon and isomers, "Create three-dimensional models of alkanes, alkenes, and alkynes using simulation." For this lab you can use MOLVIEW - a website or an app MOLECULAR CONSTRUCTOR Molview: htts: // molview, orgl Buld one alkane, one afluene, and one alkme of your choosing, with a minimum of four carbon atbma in. each molecule. Furtheimore, conseruat two pasible isomers for qach molecult, candidering beet structural and geometricat isomers if appleable Isomers
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


