Question: Lab 3.1: Candium Introduction Average atomic mass is a weighted average that depends on the number of isotopes and their respective masses. The number on

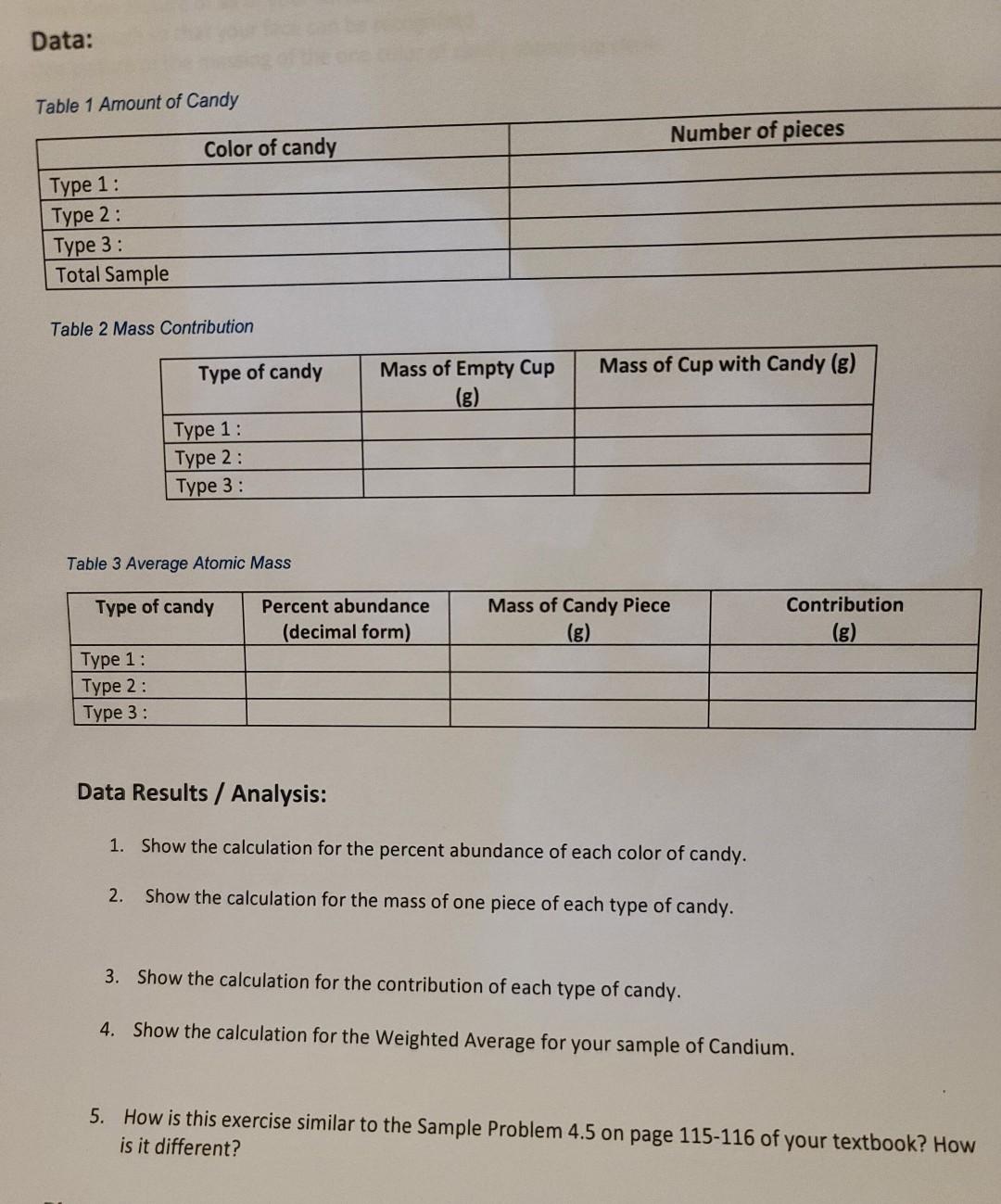
Lab 3.1: Candium Introduction Average atomic mass is a weighted average that depends on the number of isotopes and their respective masses. The number on the periodic table that you are so familiar with is a weighted average of all the known isotopes for that element. In this experiment you will use similar candies to represent the different known isotopes of a newly discovered element, Candium. You will model the process used to determine the average atomic mass number for elements. Equipment: - Mixed Sample of colored candies (M\&M's) - Paper towels - digital scale - small disposable cups (1 for each color) Setup: You will have to purchase two bags of different varieties of M\&M's (or similar candy) and mix them into a single bag/container. It is better to get varieties that have a difference in size, meaning that one is smaller than the other. Any broken pieces are not to be used for the exercise as you will be using whole pieces. Procedure: 1. You will need a small disposable cup for each color of candy in your sample bag. You will need to sort the mixture by color and count each different color of candy in your sample bag. 2. Record the number of each color of candy and total all of the candy pieces in Data Table 1. You may have to add rows to the table. 3. Using the total number of candy pieces and the number of each color of candy, calculate the abundance of each type of candy species. You will report this value as a decimal (
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


