Question: Lab Data PHASE 10 Calculate unknown concentration Metals Oxidation half-reaction Overall reaction Standard cell potential (V) Reduction half-reaction CU+2e Cu Complete the following stepe 1
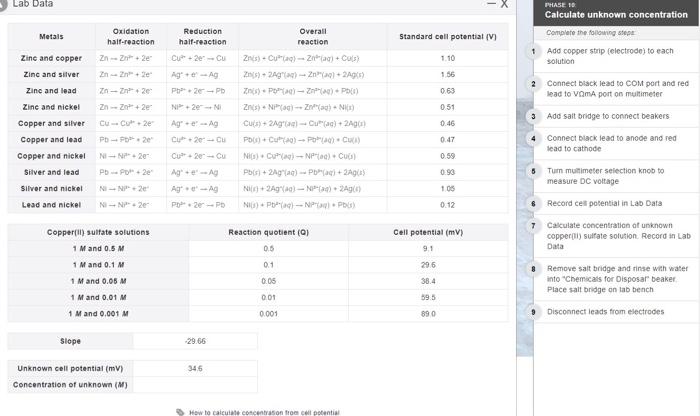
Lab Data PHASE 10 Calculate unknown concentration Metals Oxidation half-reaction Overall reaction Standard cell potential (V) Reduction half-reaction CU+2e Cu Complete the following stepe 1 Add copper strip (electrode to each solution Zn-2n+2e 1.10 Zn-2n+2e 1 56 Ag-A Ph.2 - PD Zn-2n+2e 0.63 2 Connect black lead to COM port and red lead to VomA port on multimeter ZnZn +2e NI+ 2N 0.51 0.46 Zine and copper Zinc and silver Zinc and lead Zinc and nickel Copper and silver Copper and lead Copper and nickel Silver and lead Silver and nickel Lead and nickel Ag - Ag C20-CU Znis). Cura) - Z) + Cos) 2). 2) - 2n) +2A) Zn). P-PE) Zn). NP) Cuis+ 2Ag - Ouac) + 2A) PC) - PC) Ni[1] + D ag)- NP |ad) + Cup ) Pb(s) + 2Aga) - PA NA) - NP 2A) NI) Pb) - NPP Cu-CO2e PD-2 N-P2 PD P2 9 Add salt bridge to connect beakers Connect black lead to anode and red lead to cathode 0.47 C20-CU 0.59 0.93 105 Turn multimeter selection knob to measure DC voltage N-2 Ag-A N-N2e P2-PO 0.12 Record cell potential in Lab Data Reaction quotient (0) 7 Calculate concentration of unknown copper il sulfate solution Record in Lab Data 0.5 Copperfil) sulfate solutions 1 M and 0.5M 1 M and 0.1 M 1 M and 0.05 M Cell potential (mv) 9.1 29,6 0.1 0.05 38.4 8 Remove salt bridge and rinse with water into "Chemicals for Disposar beaker Place salt bridge on tab bench 0.01 39.5 1 M and 0.01 M 1 M and 0.001 M 0.001 890 Disconnect leads from electrodes Slope 29.66 345 Unknown cell potential (mv) Concentration of unknown (M) How to calculate Concentrason from cell potential
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


