Question: using the info provided how do i find the initial concentrations of the reactants and then how do i find the initial rate in mM/s
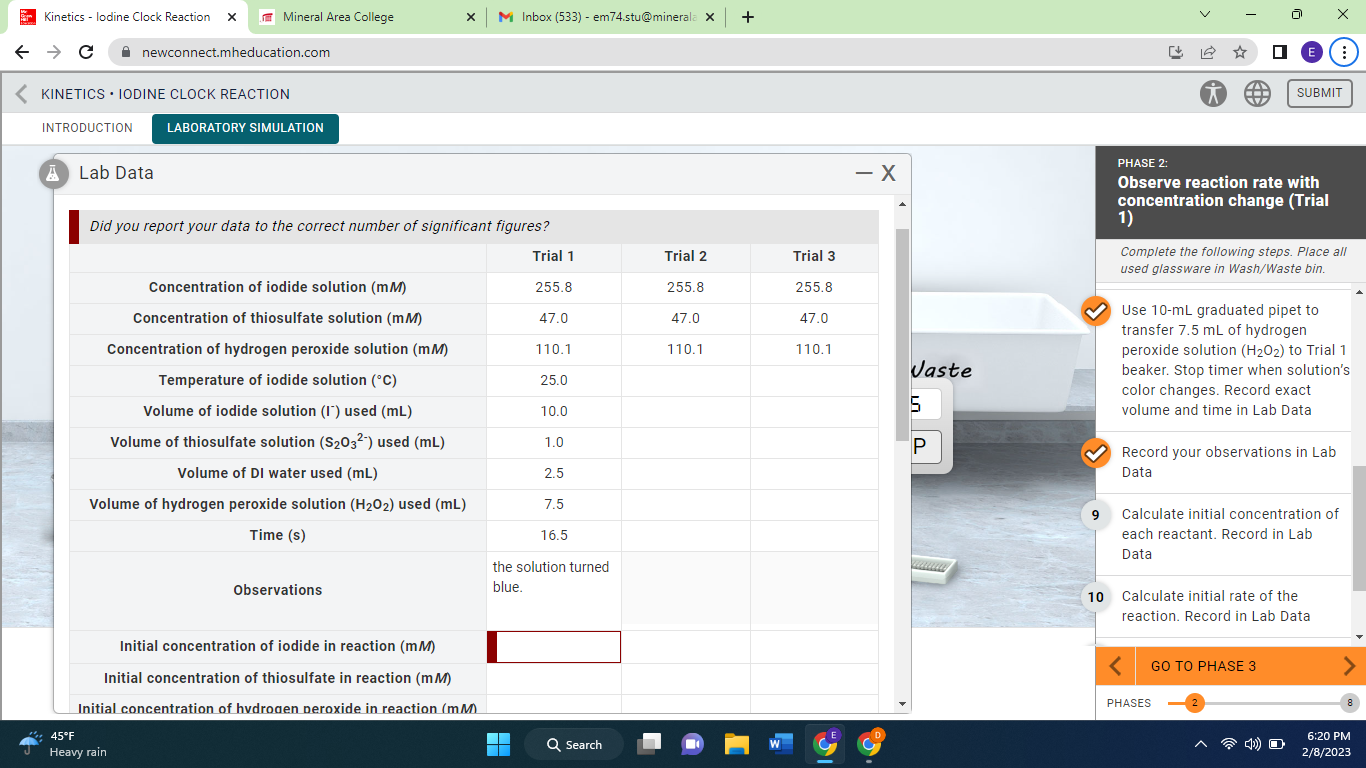
using the info provided how do i find the initial concentrations of the reactants and then how do i find the initial rate in mM/s
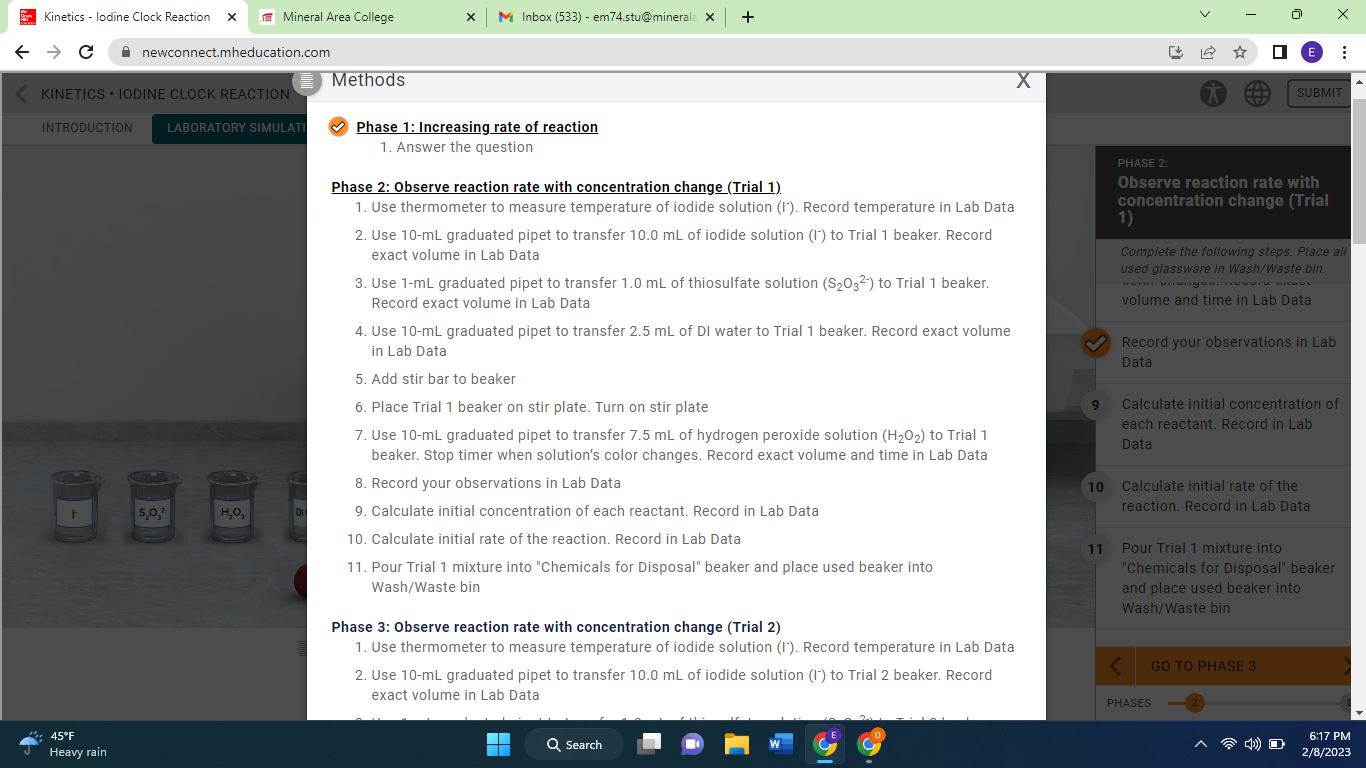
Phase 1: Increasing rate of reaction 1. Answer the question Phase 2: Observe reaction rate with concentration change (Trial 1). 1. Use thermometer to measure temperature of iodide solution (I-). Record temperature in Lab Data 2. Use 10-mL graduated pipet to transfer 10.0mL of iodide solution (I-) to Trial 1 beaker. Record exact volume in Lab Data 3. Use 1-mL graduated pipet to transfer 1.0mL of thiosulfate solution (S2O32) to Trial 1 beaker. Record exact volume in Lab Data 4. Use 10-mL graduated pipet to transfer 2.5mL of DI water to Trial 1 beaker. Record exact volume in Lab Data 5. Add stir bar to beaker 6. Place Trial 1 beaker on stir plate. Turn on stir plate 7. Use 10-mL graduated pipet to transfer 7.5mL of hydrogen peroxide solution (H2O2) to Trial 1 beaker. Stop timer when solution's color changes. Record exact volume and time in Lab Data 8. Record your observations in Lab Data 9. Calculate initial concentration of each reactant. Record in Lab Data 10. Calculate initial rate of the reaction. Record in Lab Data 11. Pour Trial 1 mixture into "Chemicals for Disposal" beaker and place used beaker into Wash/Waste bin Phase 3: Observe reaction rate with concentration change (Trial 2) 1. Use thermometer to measure temperature of iodide solution (I-). Record temperature in Lab Data 2. Use 10-mL graduated pipet to transfer 10.0mL of iodide solution (I-) to Trial 2 beaker. Record exact volume in Lab Data Phase 1: Increasing rate of reaction 1. Answer the question Phase 2: Observe reaction rate with concentration change (Trial 1). 1. Use thermometer to measure temperature of iodide solution (I-). Record temperature in Lab Data 2. Use 10-mL graduated pipet to transfer 10.0mL of iodide solution (I-) to Trial 1 beaker. Record exact volume in Lab Data 3. Use 1-mL graduated pipet to transfer 1.0mL of thiosulfate solution (S2O32) to Trial 1 beaker. Record exact volume in Lab Data 4. Use 10-mL graduated pipet to transfer 2.5mL of DI water to Trial 1 beaker. Record exact volume in Lab Data 5. Add stir bar to beaker 6. Place Trial 1 beaker on stir plate. Turn on stir plate 7. Use 10-mL graduated pipet to transfer 7.5mL of hydrogen peroxide solution (H2O2) to Trial 1 beaker. Stop timer when solution's color changes. Record exact volume and time in Lab Data 8. Record your observations in Lab Data 9. Calculate initial concentration of each reactant. Record in Lab Data 10. Calculate initial rate of the reaction. Record in Lab Data 11. Pour Trial 1 mixture into "Chemicals for Disposal" beaker and place used beaker into Wash/Waste bin Phase 3: Observe reaction rate with concentration change (Trial 2) 1. Use thermometer to measure temperature of iodide solution (I-). Record temperature in Lab Data 2. Use 10-mL graduated pipet to transfer 10.0mL of iodide solution (I-) to Trial 2 beaker. Record exact volume in Lab Data
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


