Question: Let's take look at the vapor pressure data for a mixture of benzene (B) and acetic acid (A) at 50C given below. Calculate activity coefficients
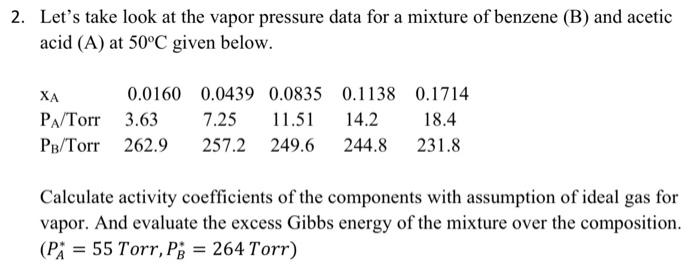
Let's take look at the vapor pressure data for a mixture of benzene (B) and acetic acid (A) at 50C given below. Calculate activity coefficients of the components with assumption of ideal gas for vapor. And evaluate the excess Gibbs energy of the mixture over the composition. (PA=55 Torr,PB=264 Torr )
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


