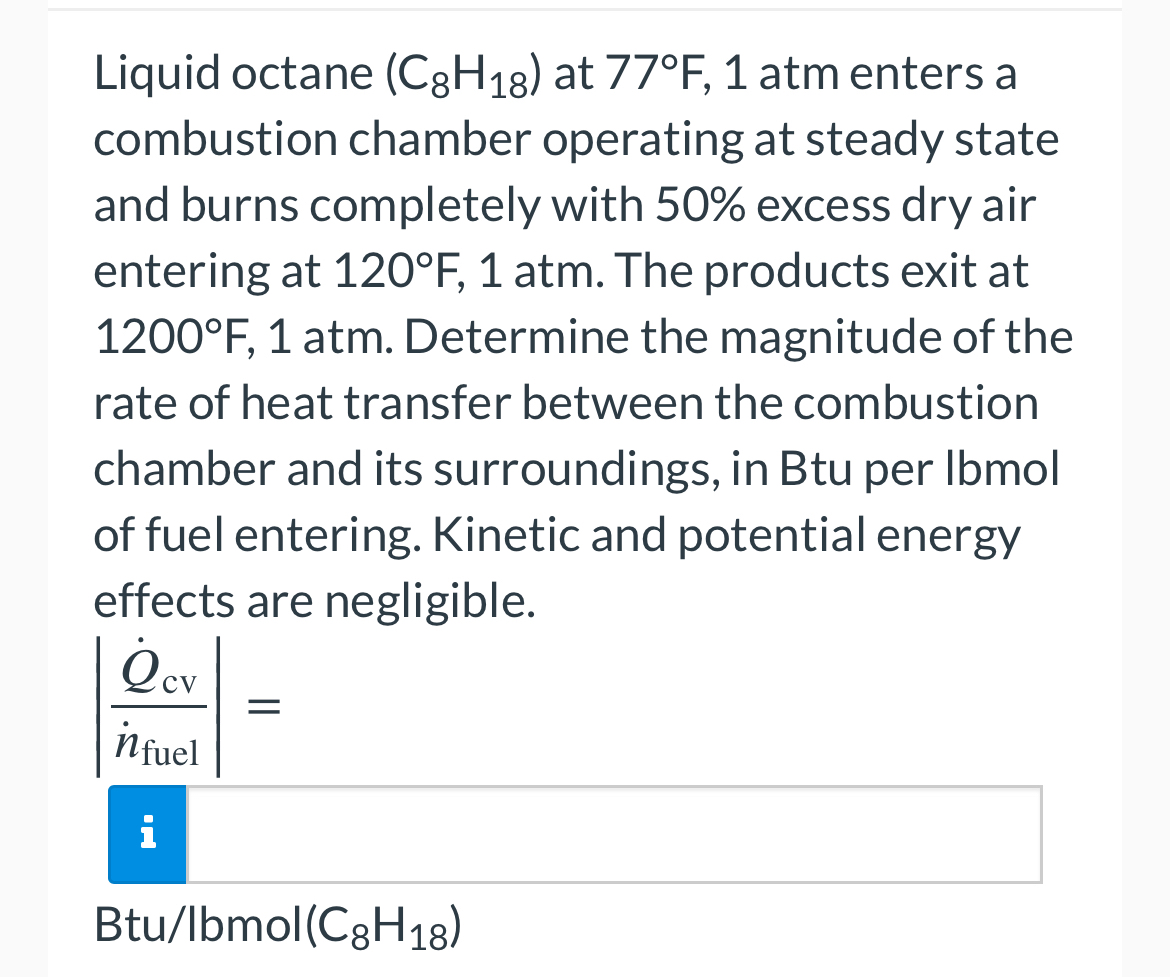
Question: Liquid octane ( C 8 H 1 8 ) at 7 7 F , 1 atm enters a combustion chamber operating at steady state and
Liquid octane at atm enters a combustion chamber operating at steady state and burns completely with excess dry air entering at atm. The products exit at atm. Determine the magnitude of the rate of heat transfer between the combustion chamber and its surroundings, in Btu per Ibmol of fuel entering. Kinetic and potential energy effects are negligible.
i
Btulbmol
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


