Question: Materials Science & Engineering Problem! (Chemical Engineering) Please answer this question as soon as possible with specific explanation. If you're going to write it in
Materials Science & Engineering Problem! (Chemical Engineering)
Please answer this question as soon as possible with specific explanation.
If you're going to write it in handwriting, please write it clearly so I can recognize it. I don't want you to write solution in cursive letter.
I can give you thumbs up when your answer is correct.
Thanks.

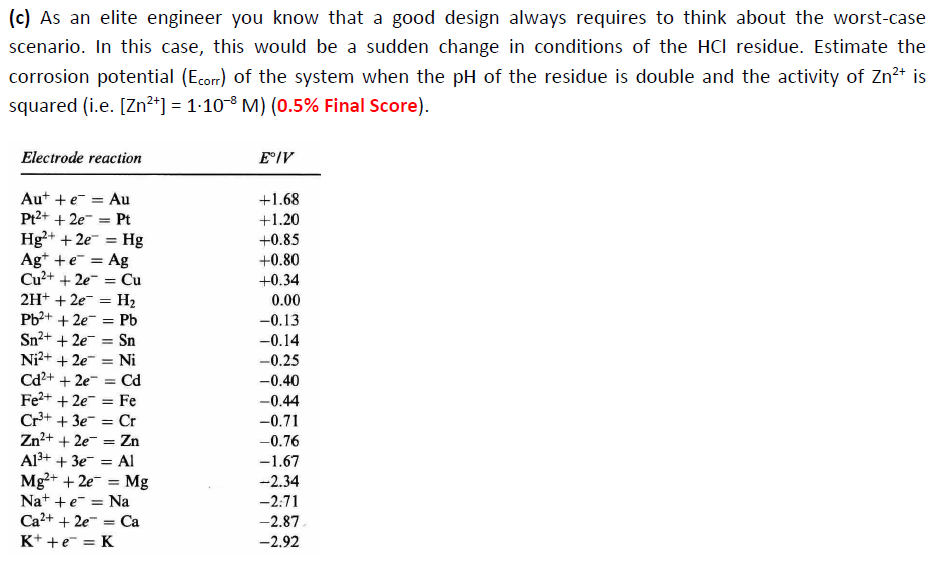
Question 1 (1.5% Final Score): You are a chemical engineer working in a factory producing hydrochloric acid (HCI). The design of the plant is old (>50 years) and there are some concerns regarding the safety of the factory. You must investigate if existing copper (Zn) pipes can be used to remove residual aqueous acid solutions collected from the HCl reacting tanks after washing. Your fantastic team performed a preliminary investigation and now you know the following findings: . Finding 1: The pH of the aqueous HCl residue is 2. Finding 2: The reacting tank is made of brass, and it leaks some Zn ions into the aqueous HCl residue solution, the concentration of which is estimated to be [Zn2+] = 1.104 M. Finding 3: The cathodic (Bc) and anodic (Ba) Tafel slopes of the corrosion process in the pipes under such conditions are estimated to be c = -0.20 V/decade of current density and Ba = 0.20 V/decade of current density. Finding 4: The exchange current densities for the cathodic and anodic reactions are iH2 = 1.10-8 A cm? and izn = 2.10-10 A cm? NB: [H*] = 1.10 PH and 1 V/decade of current density means 1 V every 10-Log(i) A cm units. With that knowledge you must: (a) Construct the Evans diagram for the corrosion of Zn under such conditions and calculate the corrosion potential (Ecorr) and the corrosion current density (icorr) of the system (NB: you can use Excel to generate the graph and you must show your reasoning with all the calculations steps, including redox reactions) (0.5% Final Score) (b) Calculate the protection current density (i protection) that you will have to apply to protect the pipeline system from corrosion (0.5% Final Score). (c) As an elite engineer you know that a good design always requires to think about the worst-case scenario. In this case, this would be a sudden change in conditions of the HCl residue. Estimate the corrosion potential (Ecorr) of the system when the pH of the residue is double and the activity of Zn2+ is squared (i.e. [Zn2+] = 1.10-8 M) (0.5% Final Score). Electrode reaction E IV = Aut + = Au Pt2+ + 2e = Pt Hg2+ + 2e = Hg Agt + = Ag Cu2+ + 2e = Cu 2H+ + 2e = H2 Pb2+ + 2e = Pb Sn2+ + 2e = Sn Ni2+ + 2e = Ni Cd2+ + 2e = Cd Fe2+ + 2e- Fe Cr3+ + 3e = Cr Zn2+ + 2e = Zn A13+ + 3e = AI Mg2+ + 2e = Mg Na+ + = Na Ca2+ + 2e = Ca K+ +e=K +1.68 +1.20 +0.85 +0.80 +0.34 0.00 -0.13 -0.14 -0.25 -0.40 -0.44 -0.71 -0.76 -1.67 -2.34 -2.71 -2.87 -2.92 = =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


