Question: Matlab program, need help to create a script to solve the following question (New to Matlab) The ideal gas law is P = nRT/V and
Matlab program, need help to create a script to solve the following question (New to Matlab)
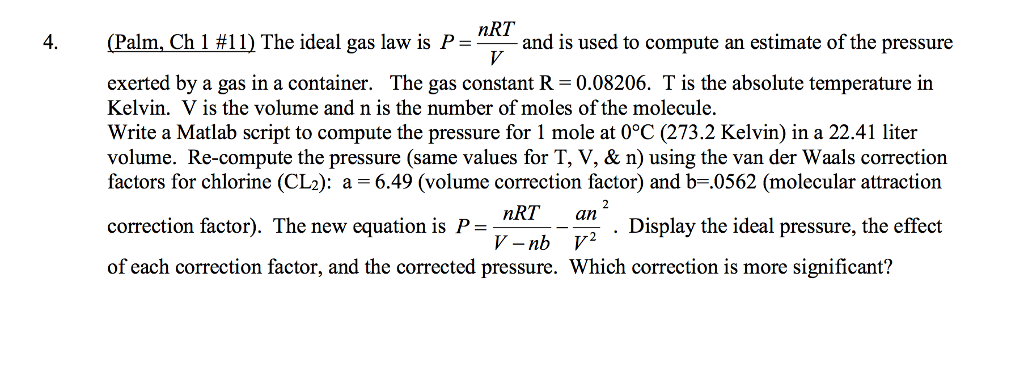
The ideal gas law is P = nRT/V and is used to compute an estimate of the pressure exerted by a gas in a container. The gas constant R = 0.08206. T is the absolute temperature in Kelvin. V is the volume and n is the number of moles of the molecule. Write a Matlab script to compute the pressure for 1 mole at 0 degree C (273.2 Kelvin) in a 22.41 liter volume. Re-compute the pressure (same values for T, V, & n) using the van der Waals correction factors for chlorine (CL_2): a = 6.49 (volume correction factor) and b = 0562 (molecular attraction correction factor). The new equation is P = nRT/V - nb - an^2/V^2. Display the ideal pressure, the effect of each correction factor, and the corrected pressure. Which correction is more significant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


