Question: may you explain and help me find the answer to both problems please. will thumbs up A student dissolves 14.86g of MgCl2(MM=95.21g/mol) in enough water
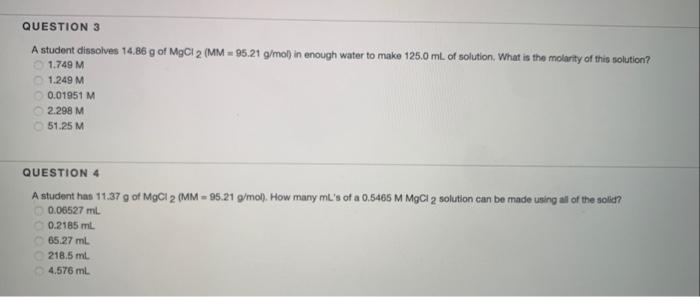
A student dissolves 14.86g of MgCl2(MM=95.21g/mol) in enough water to make 125.0mL of solution. What is the molarity of this solution? 1.749M1.249M0.01951M2.298M51.25M QUESTION 4 A student has 11.37g of MgCl2(MM=95.21g/mol). How many mL 's of a 0.5465MMgCl2 solution can be made using all of the solid? 0.06527mL0.2185mL65.27mL218.5mL4.576mL
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


