Question: Me and multiple experts so far cannot figure out B A second-order reaction has a rate constant of 0.009500/(M+5) at 30C. At 40C, the rate
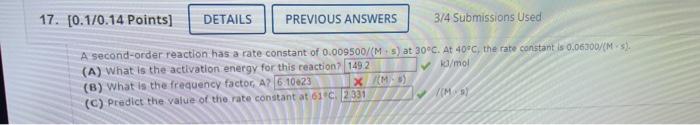
A second-order reaction has a rate constant of 0.009500/(M+5) at 30C. At 40C, the rate constant is 0.06300/(M5). (A) What is the activation energy for this reaction? ka/mos (B) What is the frequency factor, A? (C) Predict the value of the rate constant at 61+C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


