Question: Metals often form several cations with different charges. Corium, for example; forms Ce3+ and Ce4+ ions, and thallium forms Tl+ and Tl3+ ions. Cerium and
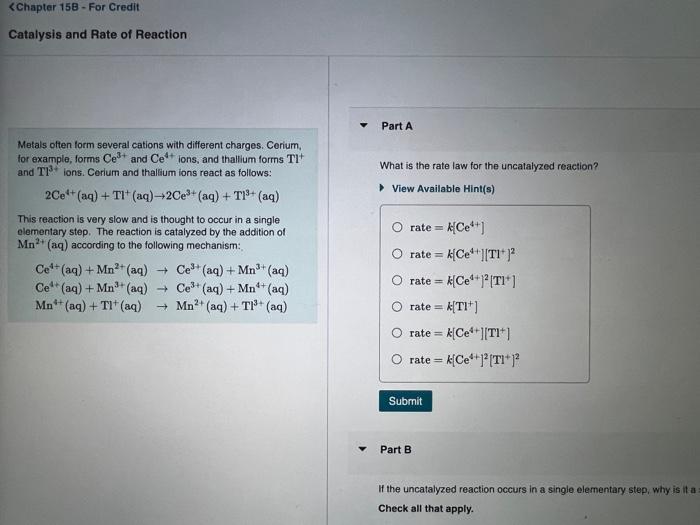
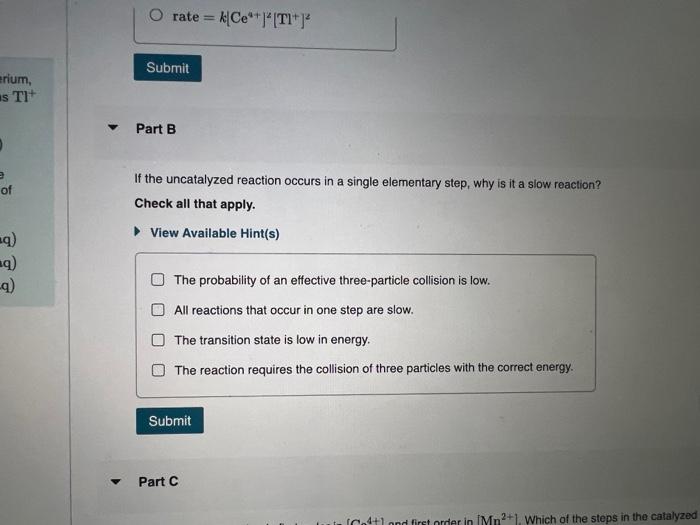
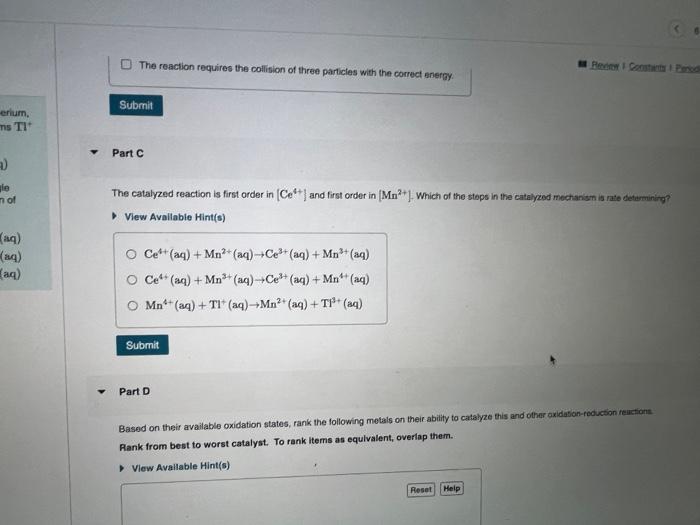
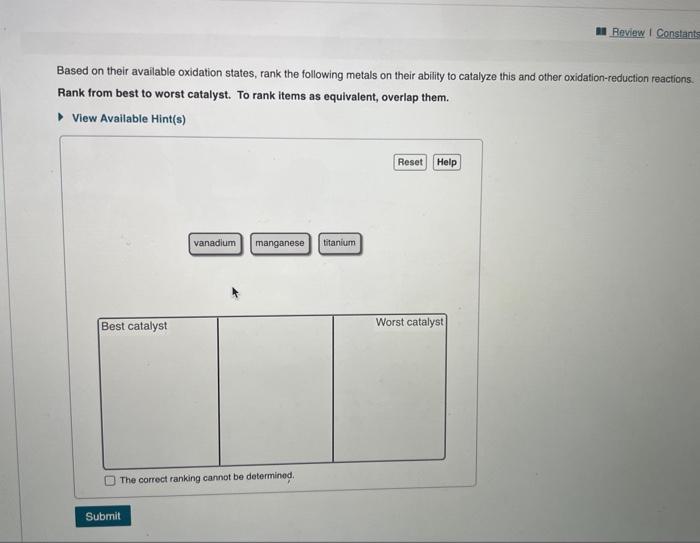
Metals often form several cations with different charges. Corium, for example; forms Ce3+ and Ce4+ ions, and thallium forms Tl+ and Tl3+ ions. Cerium and thallfum ions react as follows: What is the rate law for the uncatalyzed reaction? 2Ce4+(aq)+Tl+(aq)2Ce3+(aq)+Tl3+(aq) View Available Hint(s) This reaction is very slow and is thought to occur in a single elementary step. The reaction is catalyzed by the addition of \begin{tabular}{l|l} Mn2+(aq) according to the following mechanism: & \\ Ce4+(aq)+Mn2+(aq)Ce3+(aq)+Mn3+(aq) & rate =k[Ce4+][Tl+]2 \\ Ce4+(aq)+Mn3+(aq)Ce3+(aq)+Mn4+(aq) & rate =k[Ce4+]2[Tl+] \\ Mn4+(aq)+Tl+(aq)Mn2+(aq)+Tl3+(aq) & rate =k[Tl+] \\ & rate =k[Ce4+][Tl+] \end{tabular} Part B It the uncatalyzed reaction occurs in a single elementary step, why is it a Check all that apply. rate=k[Ce4+]2[Tl+]2 Part B If the uncatalyzed reaction occurs in a single elementary step, why is it a slow reaction? Check all that apply. View Available Hint(s) The probability of an effective three-particle collision is low. All reactions that occur in one step are slow. The transition state is low in energy. The reaction requires the collision of three particles with the correct energy. The reaction requires the collision of three particles with the correct energy. Part C The catalyzed reaction is first order in [Ce4+] and first order in [Mn2+]. Which of the stops in the catalyzed mectanisen is rate detumining? View Avallable Hint(s) Ce4+(aq)+Mn2+(aq)+Ce3+(aq)+Mn3+(aq)Ce4+(aq)+Mn3+(aq)Ce3+(aq)+Mn4+(aq)Mn4+(aq)+Tl+(aq)Mn2+(aq)+Tl3+(aq) Part D Based on their available oxidation states, rank the following metals on their ability to catalyze this and other axidation-recuction reuctions: Rank from best to worst catalyst. To rank items as equivalent, overlap them. Based on their available oxidation states, rank the following metals on their ability to catalyze this and other oxidation-reduction reactions. Rank from best to worst catalyst. To rank items as equivalent, overlap them. View Available Hint(s) The correct ranking cannot be determined
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


