Question: Methane (assumed to be in its ideal-gas state) is compressed adiabatically from 20C and 140kPa to 560KPa. The compressor efficiency is 0.75 . What is
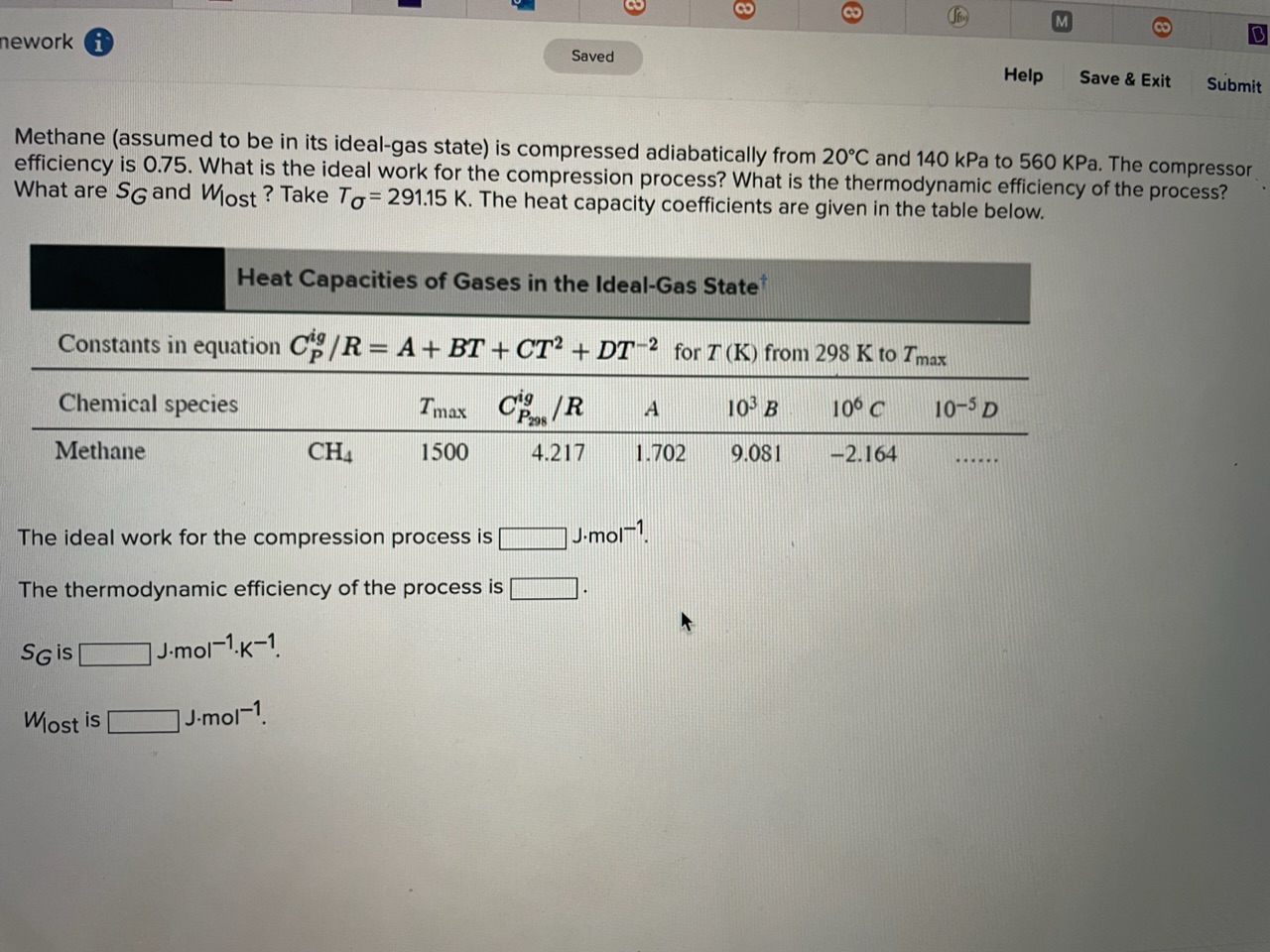
Methane (assumed to be in its ideal-gas state) is compressed adiabatically from 20C and 140kPa to 560KPa. The compressor efficiency is 0.75 . What is the ideal work for the compression process? What is the thermodynamic efficiency of the process? What are SG and Wlost ? Take T=291.15K. The heat capacity coefficients are given in the table below. Heat Capacities of Gases in the Ideal-Gas State Constants in equation CPig/R=A+BT+CT2+DT2 for T(K) from 298K to Tmax The ideal work for the compression process is Jmol1. The thermodynamic efficiency of the process is SG is Jmol1K1 Wost is Jmol1
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


