Question: Methane (assumed to be in its ideal-gas state) is compressed adiabatically from 20C and 140kPa to 560KPa. The compressor efficiency is 0.75 . What is
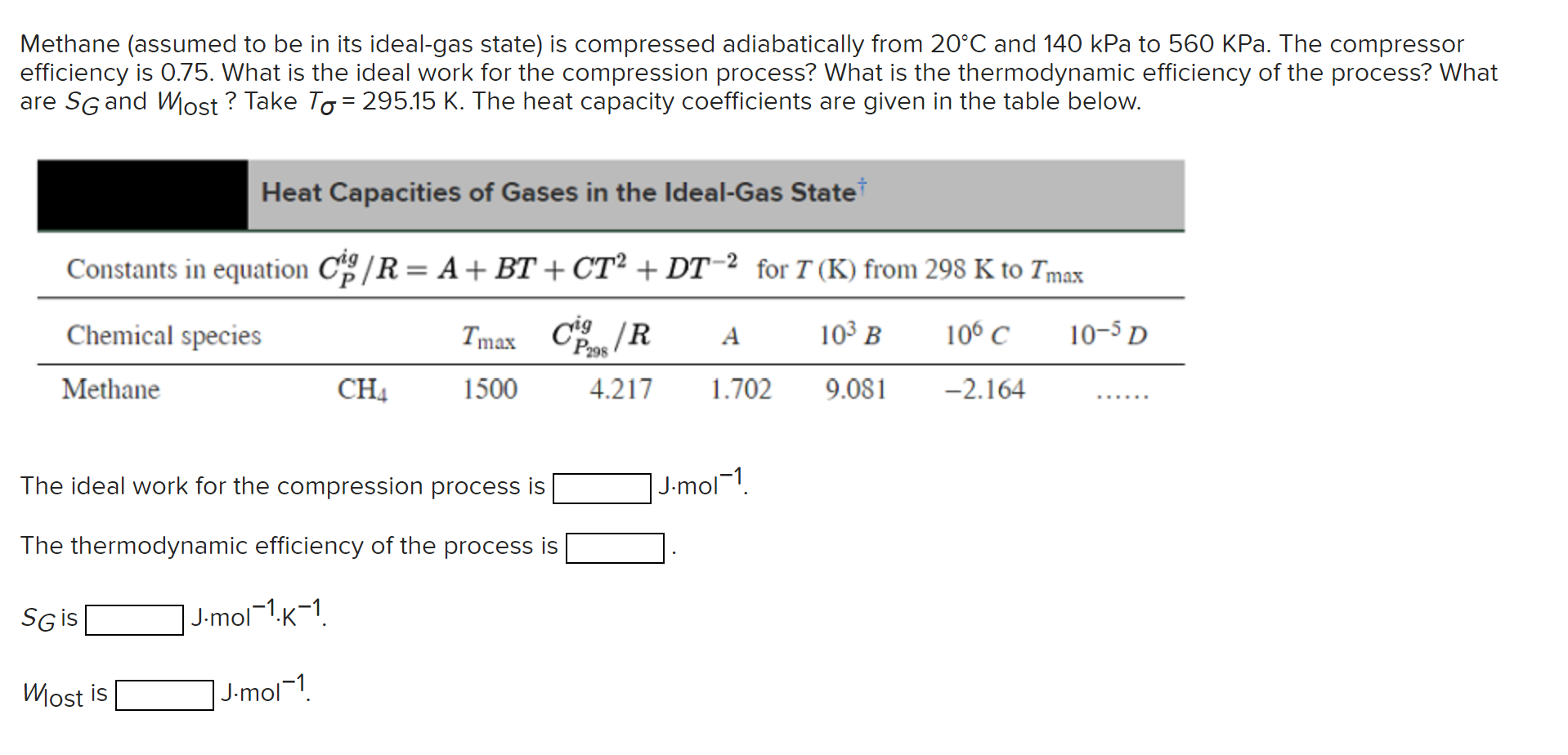
Methane (assumed to be in its ideal-gas state) is compressed adiabatically from 20C and 140kPa to 560KPa. The compressor efficiency is 0.75 . What is the ideal work for the compression process? What is the thermodynamic efficiency of the process? What are SG and Wlost ? Take T=295.15K. The heat capacity coefficients are given in the table below. Heat Capacities of Gases in the Ideal-Gas State Constants in equation CPig/R=A+BT+CT2+DT2 for T(K) from 298K to Tmax The ideal work for the compression process is Jmol1. The thermodynamic efficiency of the process is SG is ]J2mol1K1 Wlost is Jmol1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


