Question: Methane (CH4) and chlorine (Cl2) react to form dichloromethane (CH2Cl2) and hydrochloric acid (HCl). A stoichiometric mixture (fresh feed) of methane and chlorine flows at
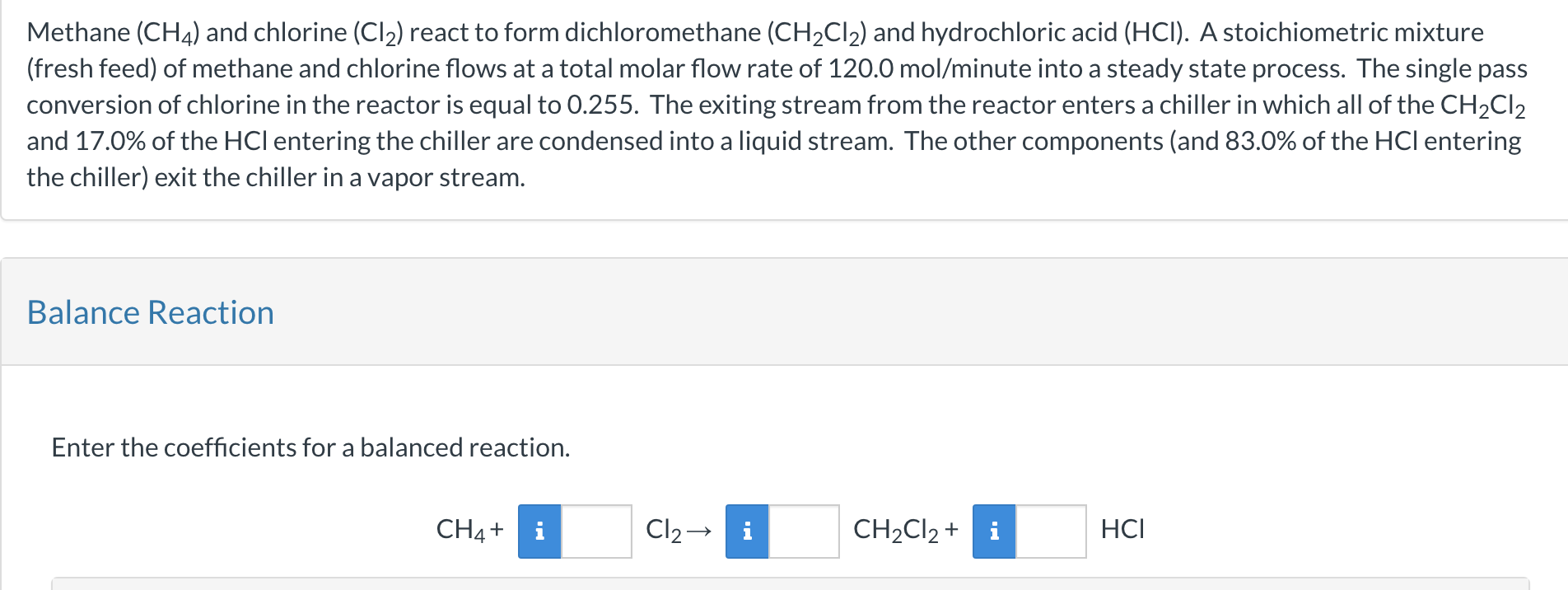
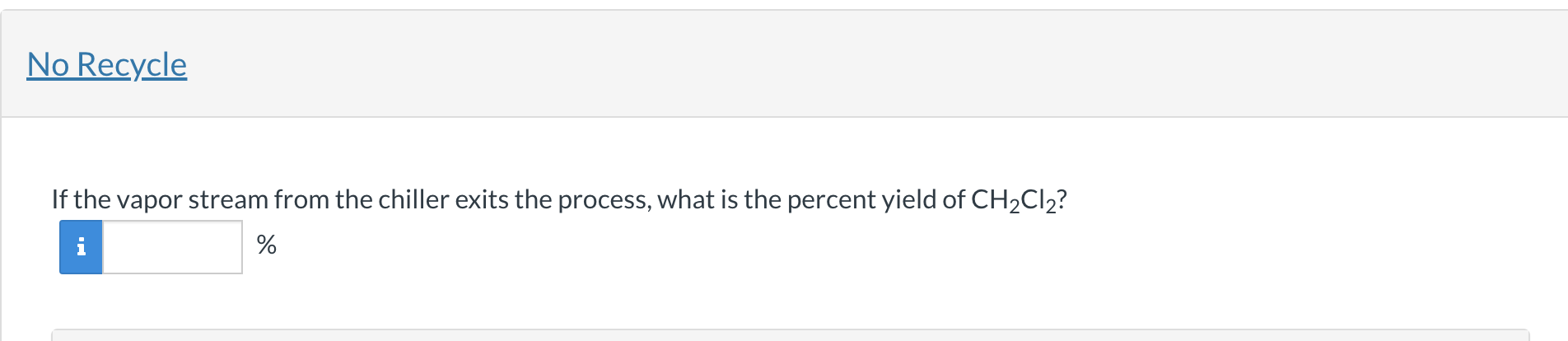
Methane (CH4) and chlorine (Cl2) react to form dichloromethane (CH2Cl2) and hydrochloric acid (HCl). A stoichiometric mixture (fresh feed) of methane and chlorine flows at a total molar flow rate of 120.0mol/minute into a steady state process. The single pass conversion of chlorine in the reactor is equal to 0.255. The exiting stream from the reactor enters a chiller in which all of the CH2Cl2 and 17.0% of the HCl entering the chiller are condensed into a liquid stream. The other components (and 83.0% of the HCl entering the chiller) exit the chiller in a vapor stream. Balance Reaction Enter the coefficients for a balanced reaction. CH4+Cl2CH2Cl2+HCl If the vapor stream from the chiller exits the process, what is the percent yield of CH2Cl2 ? %
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


