Question: please answer parts a),b), and c) Please use the molecular balance method for this problem Methane (CH4) and chlorine (Cl2) react to form dichloromethane (CH2Cl2)
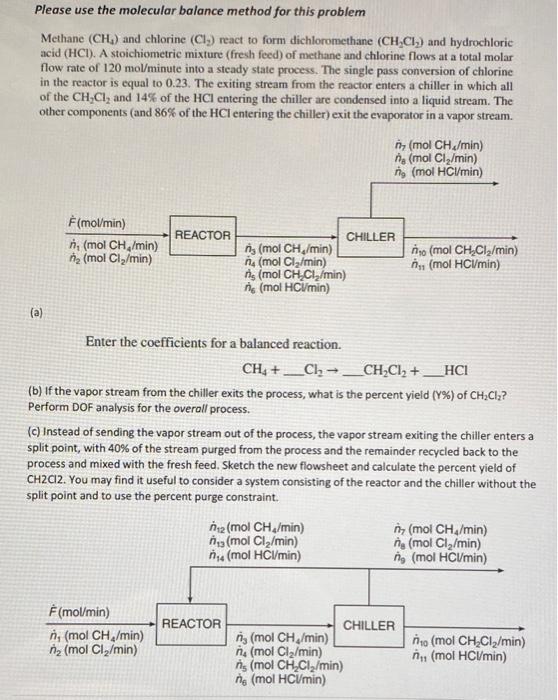
Please use the molecular balance method for this problem Methane (CH4) and chlorine (Cl2) react to form dichloromethane (CH2Cl2) and hydrochloric acid (HCl). A stoichiometric mixture (fresh feed) of methane and chlorine flows at a total molar flow rate of 120mol/minute into a steady state process. The single pass conversion of chlorine in the reactor is equal to 0.23 . The exiting stream from the reactor enters a chiller in which all of the CH2Cl2 and 14% of the HCl entering the chiller are condensed into a liquid stream. The other components (and 86% of the HCl entering the chiller) exit the evaporator in a vapor stream. (a) Enter the coefficients for a balanced reaction. CH4+Cl2CH2Cl2+HCl (b) If the vapor stream from the chiller exits the process, what is the percent yield (Y%) of CH2Cl2 ? Perform DOF analysis for the overall process. (c) Instead of sending the vapor stream out of the process, the vapor stream exiting the chiller enters a split point, with 40% of the stream purged from the process and the remainder recycled back to the process and mixed with the fresh feed. Sketch the new flowsheet and calculate the percent yield of CH2Cl2. You may find it useful to consider a system consisting of the reactor and the chiller without the split point and to use the percent purge constraint
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


