Question: methane mol% = 0.56 & ethylene mol%= 0.34 a. BUBL P at T= 222.15K b. DEW P at T = 222.15K Assuming the validity of
methane mol% = 0.56 & ethylene mol%= 0.34
a. BUBL P at T= 222.15K b. DEW P at T = 222.15K
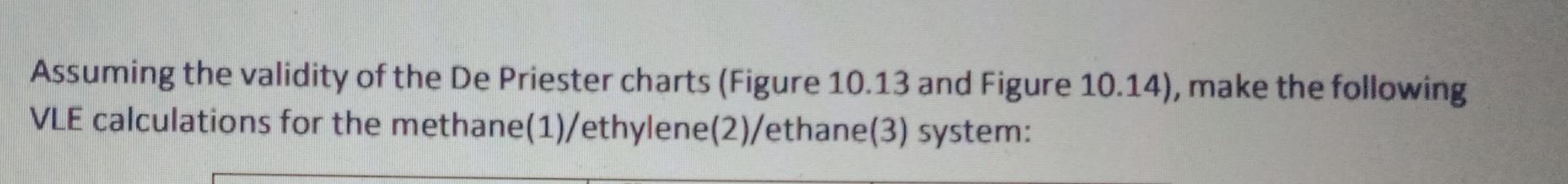
Assuming the validity of the De Priester charts (Figure 10.13 and Figure 10.14), make the following VLE calculations for the methane(1)/ethylene(2)/ethane(3) system
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


