Question: MisseD THIS? Read Section 14.5 (Page) ; Walch KCV t4. .5 ME 14.4. AnaqueousKNO3solutionismadeusing75.9gofKNO3dilutedtoatolalsolutionvolumeof1.60L.(Assumeadensityof1.06g/mLforthesolution.)Caloulatethemolarityofthesalution.Expressthemolaritytothreesignificantfigures. Correct Molarity can be cakulaled by driding the amount of the
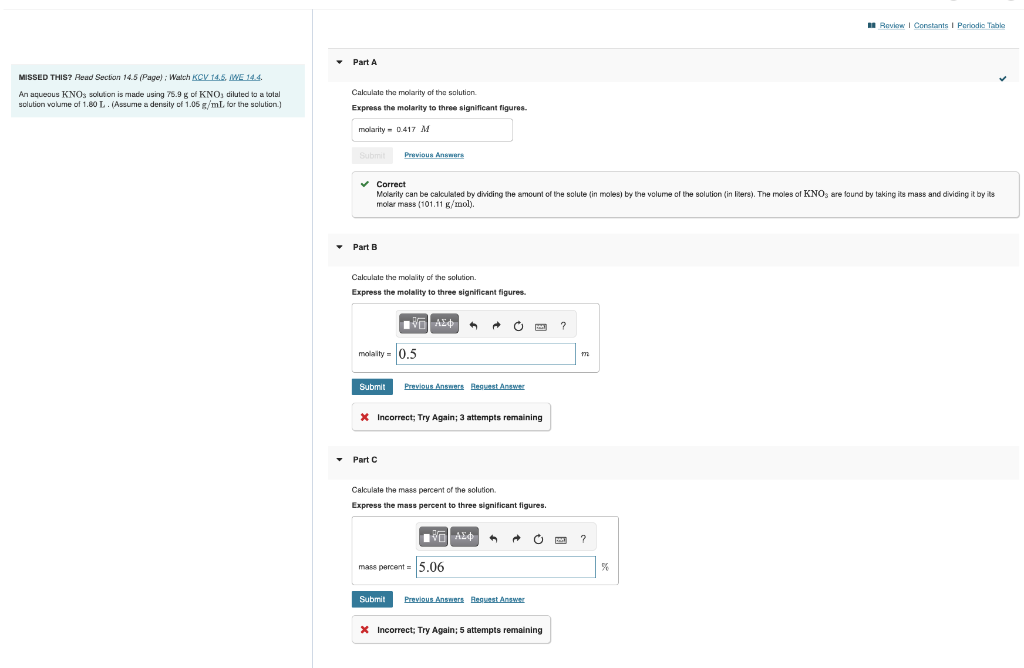
MisseD THIS? Read Section 14.5 (Page) ; Walch KCV t4. .5 ME 14.4. AnaqueousKNO3solutionismadeusing75.9gofKNO3dilutedtoatolalsolutionvolumeof1.60L.(Assumeadensityof1.06g/mLforthesolution.)Caloulatethemolarityofthesalution.Expressthemolaritytothreesignificantfigures. Correct Molarity can be cakulaled by driding the amount of the solute (in moles) by the volume of the solution (in lisers). The moles of KNO are lound by taking its mass and divicing it by its molar mass {101.11g/mol). Part B Caloulate the molality of the solution. Express the molality to three significant figures. 4. Incorrect; Try Again; 3 attempts remaining - Part C Calculate the mass percent of the solution. Express the mass percent to three significant figures. Incorrect; Try Again; 5 attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


