Question: Part A MISSED THIS? Read Section 14.5 (Pages 594 - 601) ; Watch KCV 14.5. IWE 14.4 A solution is prepared by dissolving 28.4 g
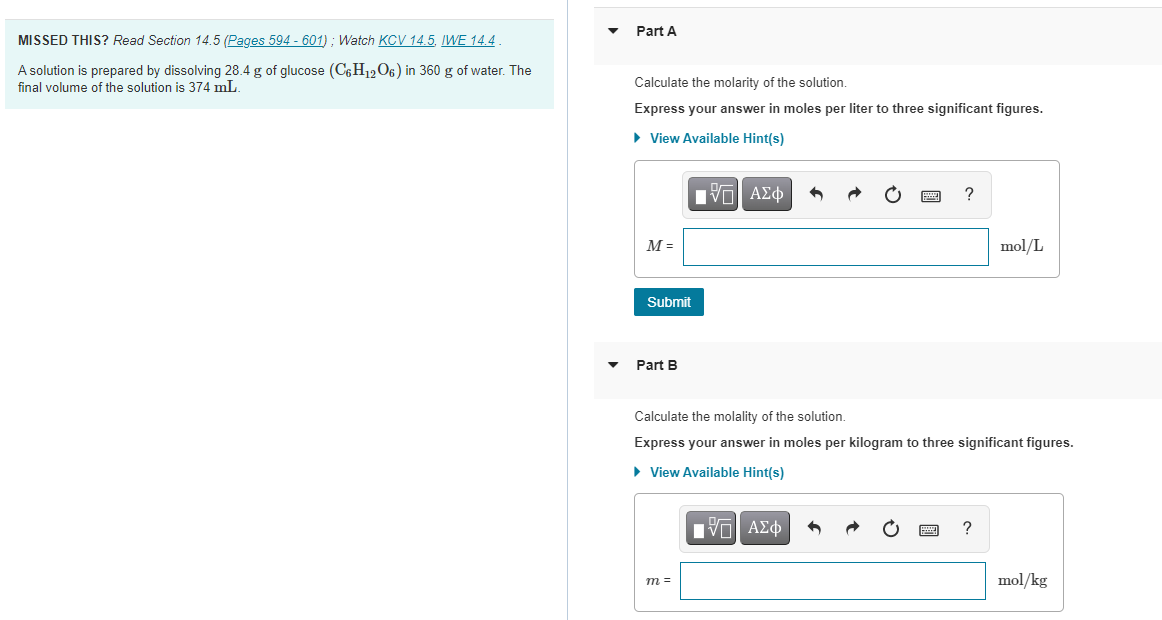
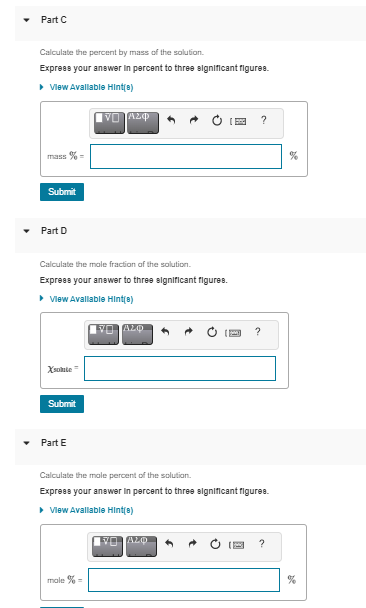
Part A MISSED THIS? Read Section 14.5 (Pages 594 - 601) ; Watch KCV 14.5. IWE 14.4 A solution is prepared by dissolving 28.4 g of glucose (C6H1206) in 360 g of water. The final volume of the solution is 374 mL. Calculate the molarity of the solution. Express your answer in moles per liter to three significant figures. View Available Hint(s) ? M = mol/L Submit Part B Calculate the molality of the solution. Express your answer in moles per kilogram to three significant figures. View Available Hint(s) IVO AEO ? ms mol/kg Part C Calculate the percent by mass of the solution Express your answer in percent to three significant figures. View Available Hint(0) IVO ALQ ? mass X Submit Part D Calculate the male fraction of the solution Express your answer to three significant figures. View Available Hint(e) IVOLO Xsolute Submit Part E Calculate the male percent of the solution. Express your answer in percent to three significant figuree. View Available Hinte) ? male % %
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


