Question: MISSED THIS? Read Section 16.8 (Pages.701 - 710); Watch KCV 16.8. IWE 16,12. Calculate the equilibrium partial pressure of CO2. Consider the following reaction: Express
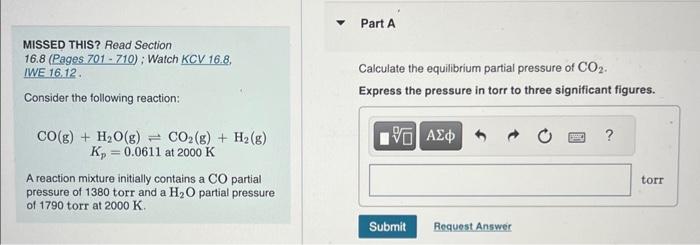
MISSED THIS? Read Section 16.8 (Pages.701 - 710); Watch KCV 16.8. IWE 16,12. Calculate the equilibrium partial pressure of CO2. Consider the following reaction: Express the pressure in torr to three significant figures. CO(g)+H2O(g)CO2(g)+H2(g)Kp=0.0611at2000K A reaction mixture initially contains a CO partial pressure of 1380 torr and a H2O partial pressure of 1790 torr at 2000K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


