Question: MisSED THIS? Read Section 16.8 (Pagos.701 - 710) : Watch KCV 16.8. IWE 16.12. Calculate the equalbrium partial pressure of CO2. Consider the following reaction:
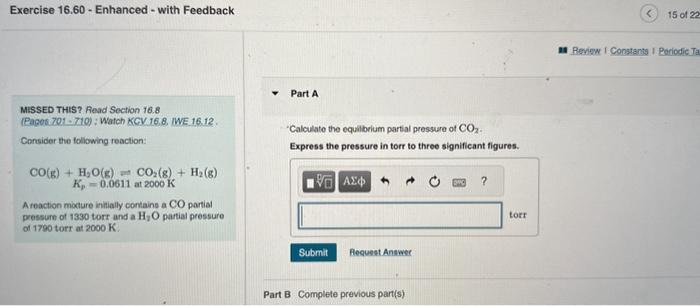
MisSED THIS? Read Section 16.8 (Pagos.701 - 710) : Watch KCV 16.8. IWE 16.12. Calculate the equalbrium partial pressure of CO2. Consider the following reaction: Express the pressure in torr to three significant figures. CO(g)+Kp=0.0611at2000KH2O(g)CO2(g)K+H2(g) A reaction moture intiaily containe a CO partial pressure of 1330 torr and a H2O partial pressure of 1790 torr at 2000K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


