Question: Model 4 - Dilutions M,V=M,V, 9 ml DH, 1 ml stock solution IM 0.1 M dis Stock solution 1/10 dilution of stock solution Sometimes solutions
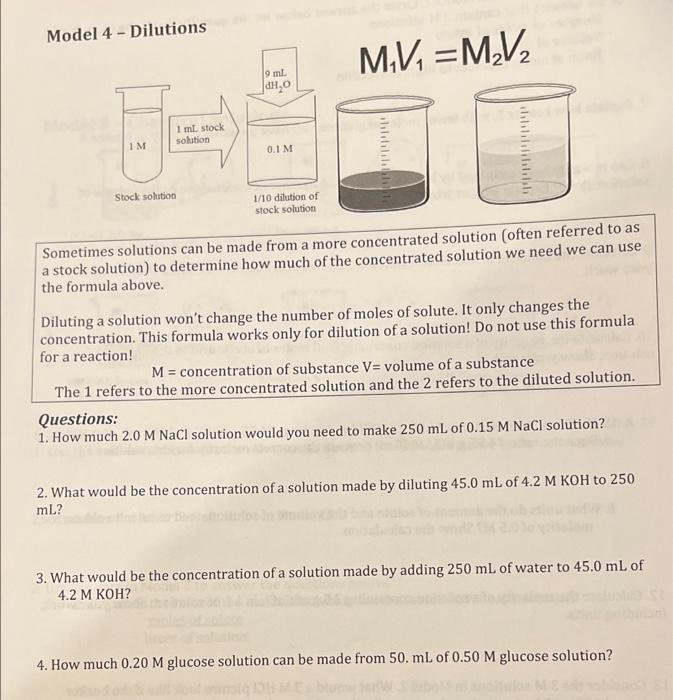
Model 4 - Dilutions M,V=M,V, 9 ml DH, 1 ml stock solution IM 0.1 M dis Stock solution 1/10 dilution of stock solution Sometimes solutions can be made from a more concentrated solution (often referred to as a stock solution) to determine how much of the concentrated solution we need we can use the formula above. Diluting a solution won't change the number of moles of solute. It only changes the concentration. This formula works only for dilution of a solution! Do not use this formula for a reaction! M = concentration of substance V= volume of a substance The 1 refers to the more concentrated solution and the 2 refers to the diluted solution. Questions: 1. How much 2.0 M NaCl solution would you need to make 250 mL of 0.15 M NaCl solution? 2. What would be the concentration of a solution made by diluting 45.0 mL of 4.2 M KOH to 250 ml? 3. What would be the concentration of a solution made by adding 250 mL of water to 45.0 mL of 4.2 M KOH? 4. How much 0.20 M glucose solution can be made from 50. mL of 0.50 M glucose solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


