Question: Model Gasoline as Octane (CH8) 18 Model air as 3.76 moles of Nitrogen (N) for every 1 mole of Oxygen (0) With these assumptions
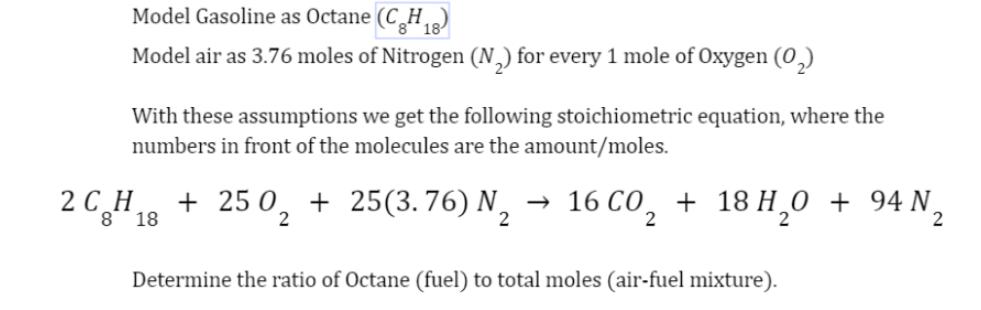
Model Gasoline as Octane (CH8) 18 Model air as 3.76 moles of Nitrogen (N) for every 1 mole of Oxygen (0) With these assumptions we get the following stoichiometric equation, where the numbers in front of the molecules are the amount/moles. 2 CH 18 8 + 250, +25(3.76) N 16C0, + 18H,0 + 94N, 2 2 Determine the ratio of Octane (fuel) to total moles (air-fuel mixture).
Step by Step Solution
There are 3 Steps involved in it
The stoichiometric equation for the combustion of octane C8H18 with oxygen O2 and ... View full answer

Get step-by-step solutions from verified subject matter experts


