Question: Module 3 1. Reaction between Brand H2O2 in acidic medium is as follows 2Br + H2O2 + 2H* Br2 + 2H2O The proposed mechanism is
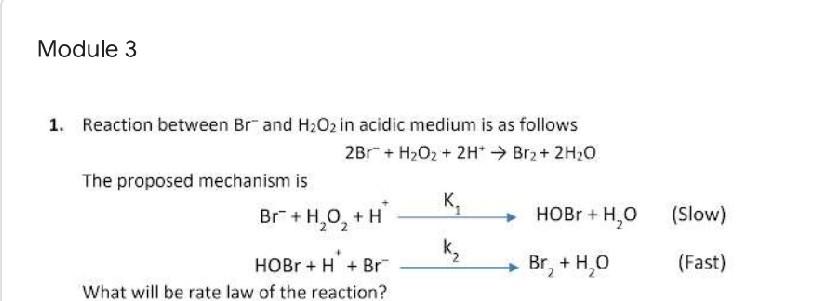
Module 3 1. Reaction between Brand H2O2 in acidic medium is as follows 2Br + H2O2 + 2H* Br2 + 2H2O The proposed mechanism is K. Br +H,0, +H HOBr + H+ Br K Br +HO What will be rate law of the reaction? HOBr + H, (Slow) (Fast)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


