Question: Module 4 1. (a) Assuming the 18-electron rule to be valid, calculate the number of M-M bonds in Os,(CO)is and 4+3=7 [Fe (CO) 12(143.CO)] (b)
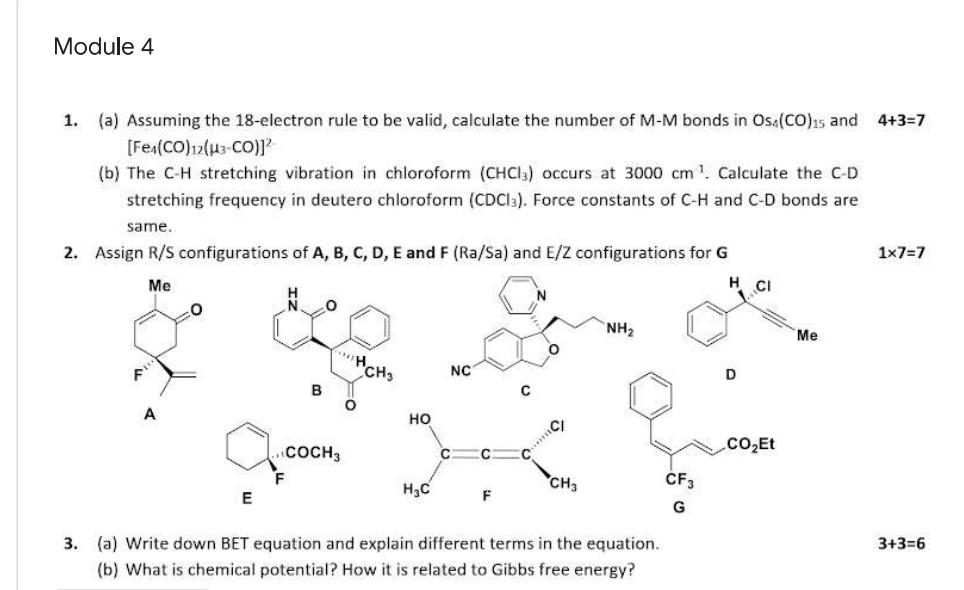
Module 4 1. (a) Assuming the 18-electron rule to be valid, calculate the number of M-M bonds in Os,(CO)is and 4+3=7 [Fe (CO) 12(143.CO)] (b) The C-H stretching vibration in chloroform (CHCI:) occurs at 3000 cm. Calculate the C-D stretching frequency in deutero chloroform (CDC13). Force constants of C-H and C-D bonds are same. 2. Assign R/S configurations of A, B, C, D, E and F (Ra/Sa) and E/Z configurations for G 1x7=7 Me 0 O NH2 Me HC "CH F NC D B O A HO CI CO2Et C F CF3 H3C E F G 3+3=6 3. (a) Write down BET equation and explain different terms in the equation. (b) What is chemical potential? How it is related to Gibbs free energy
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


